This technology can serve three important purposes. First, data from numerous studies indicate that imaging technologies can bring a less-experienced clinician up to the level of an expert observer, in terms of discriminating between normal eyes and those with early glaucoma. (In some studies imaging devices have done better than the experts; in others, they haven't done quite as well.)
Second, imaging technology provides information that is standardized, objective and quantitative. Obviously this is an advantage.
Third, it reduces the potential redundancy of the clinical exam. If perimetry indicates a subtle defect but you have no other supporting evidence, the literature suggests that you may have to repeat the visual field as many as six times to confirm the presence of the defect with a reasonable degree of certainty. However, if your imaging device finds a structural abnormality corresponding to the one indicated by perimetry results, it's likely that the functional defect is real. Being able to correlate the findings allows you to make faster and more reliable diagnoses of glaucomatous damage.
How Often Should You Image?
If I'm doing perimetry on a patient, I'll do imaging during the same visit to correlate structure and function. In a stable patient I'll do this at least yearly, sometimes twice a year. If someone is changing, I'll do it more frequently. Unfortunately, many insurance plans won't pay for perimetry or imaging more than once a year, so you have to either charge the patient or do it for free. Nevertheless, I think this is important for patients who may be progressing.
By themselves, imaging instruments have primarily been shown to be useful as diagnostic aids. The clinical studies supporting their value have almost entirely used them for this purpose, and the existing detection progression algorithms have not been extensively tested. However, the HRT's progression software has performed well in studies done at Dalhousie University in Halifax, Nova Scotia, so the HRT may be useful in helping to identify (or at least raise suspicion of) progression. However, I'd still correlate these data with clinical findings and perimetry.
Needless to say, you shouldn't test every patient for possible glaucoma—such a strategy wouldn't be efficient or serve the patient well. These machines produce false positives and negatives, so testing everyone would incorrectly diagnose some patients as having glaucoma or indicate the presence of an abnormality that isn't there, necessitating further workup.
Tips for Effective Use
A number of factors can affect imaging measurement and interpretation:
• Check the results by looking. It's easy to miss a subtle abnormality, but if an imaging instrument identifies a defect or abnormality in the optic nerve or nerve fiber layer, it's important to reevaluate the disc or NFL visually and make sure you can see it. Even if a defect is subtle, you should be able to identify it when you know exactly where to look. If you can't see the defect, take the imaging data with a grain of salt, unless you're a very inexperienced observer and you haven't looked at a lot of optic nerves or nerve fiber layers. (If that's the case, it's worth doing a little further study to become more proficient at the ON exam.)
• Take the normative database into account. This database determines what the machine categorizes as abnormal. In rare instances you may see an abnormality when examining the eye but find that the imaging technology didn't pick it up. If you look closely at the imaging data, you'll probably find that an abnormality was recorded, but didn't fall in the abnormal range defined by the database.
There's no reason to be timid about using the normative database; just remember that it may not always confirm your visual diagnosis. If your observation isn't supported by the imaging instrument, assume the abnormality is there and the imaging device is in error.
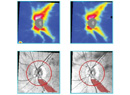
 |
| Data for the right eye of a patient with a superior arcuate visual field defect. Imaging shows more damage than the visual field reveals, with superior as well as inferior optic nerve/RNFL findings. |
• Pay attention to image quality. Remember to make sure the scan is of good enough quality to analyze. The Stratus OCT (Carl Zeiss Meditec), GDx VCC (Laser Diagnostic Technologies) and HRT give you a number that represents either standard deviation of measurements or signal strength—some parameter that indicates the quality of the image. The OCT indicates a high-quality image by a signal strength greater than 6. Also, the image should be evenly bright across the scan, without areas of dropout or missing signal. When using the HRT, you want to have a mean pixel height standard deviation of less than 50—preferably less than 30. When using the GDx VCC you want to have a Numerical Quality Score equal to or greater than 8. Also, if the GDx image looks like a sunburst, with bright colors spreading in all directions from the optic nerve, the image and analysis should not be used.
• Image registration. When repeating an earlier scan or using it to derive numerical data, check the alignment. For example, for the OCT RNFL scan, and GDx and HRT scans, make sure that the optic nerve is centered as well as possible in the image.
• Analysis quality. Make sure the analysis algorithm is working properly as it assesses the image. Unfortunately, there is no parameter that will always tell you whether or not the analysis worked. The OCT will give a message indicating "Analysis Confidence Low" in some cases, but you really need to look at the image yourself. If the white lines that define the tissue borders don't follow the actual edges of the tissue—if they go up into the vitreous or down into the choroid, or if the two white lines meet for more than 10 percent of the scan—then the analysis is likely to be faulty. On the GDx, if the nasal RFNL is the thickest region, the analysis should be taken with a grain of salt. If the HRT displays of cross-sections through the optic nerve are very jagged, with many peaks and troughs, then the analysis should be questioned.
• Instrument calibration. Have your instrument checked out periodically by company service personnel to make sure it's measuring accurately.
Instrument Pros and Cons
In terms of which of the commercially available instruments might be most productive in this capacity, the OCT, GDx and HRT are about equally useful; none of them will provide useful data for every patient.
The HRT does a good job of looking at the topography of the optic nerve, but because of the way it defines the neuroretinal rim, blood vessels are sometimes included in the measurement. Also, patients with very small or large discs may confound it. On the other hand, it does have software for monitoring progression.
Nerve fiber analysis is more specifically performed by the GDx and the OCT. GDx has the ability to identify birefringent tissue in the eye, and the software compensation for non-RNFL birefringence is better than ever. However, the measurement from the anterior and posterior segments can still be confounded, and in some percentage of patients a GDx scan will be "atypical," indicating excess birefringence found in unexpected places. OCT technology does a good job of providing cross-sectional analysis of the tissue, but it can be thrown off in eyes that are highly myopic.
Ultimately, the best choice for a given practice depends what you want to get out of the instrument. (Remember that we're only discussing detecting glaucoma here, not evaluating the macula or anterior segment.)
Technology Worth Using
For most clinicians, including imaging technology in the protocol when diagnosing glaucoma can be a significant advantage. However, it's important to remember that imaging technology shouldn't be used in a vacuum. Data from these instruments need to be integrated with the results of your clinical exam, as well as functional assessment through perimetry. Used carefully and in the proper context, they can do a lot to help identify which of your patients are healthy, and which are already showing signs of damage.
Dr. Schuman is Eye and Ear Foundation professor and chairman of the department of ophthalmology at the University of Pittsburgh school of medicine, and director of UPMC Eye Center. He is also professor of bioengineering at the University of Pittsburgh. He receives research funding from Carl Zeiss Meditec and Heidelberg Engineering, as well as the National Institutes of Health, for studies of technologies for glaucoma diagnostics. He also receives royalties for intellectual property licensed by the Massachusetts Institute of Technology to Carl Zeiss Meditec.