For the moment, combination therapy utilizing available pharmacotherapies with macular laser appears to be the treatment of choice for diabetic retinopathy.
Diabetic retinopathy is one of the leading causes of new cases of blindness among adults age 20 to 74 years in the United States.1 Estimates have projected that by 2050, the number of Americans 40 years or older with DR and vision-threatening diabetic retinopathy will triple, from 5.5 million in 2005 to 16 million for DR, and from 1.2 million in 2005 to 3.4 million for vision-threatening DR. Increases among those 65 years or older will be more pronounced (2.5 million to 9.9 million for DR and 0.5 million to 1.9 million for vision-threatening DR).2 These figures underscore the need for effective treatments for complications of diabetic retinopathy.
Proliferative DR
 |
 |
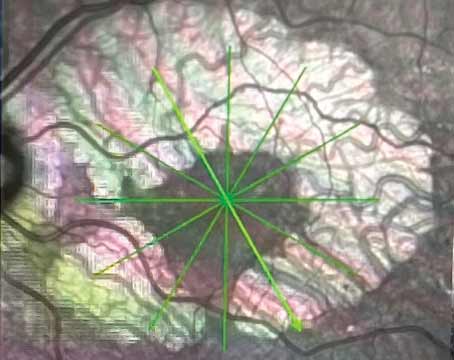
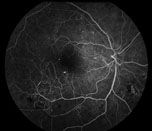
| Figure 1. Fluorescein angiogram of the right eye with severe non-proliferative diabetic retinopathy demonstrating intra-retinal microvascular abnormalities, or IRMA. | Figure 2. Color fundus photo of the right eye with proliferative diabetic retinopathy shows florid neovascularization of the retina. |
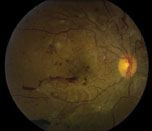
Proliferative diabetic retinopathy, a stage that is associated with severe vision loss, is characterized by the development of abnormal blood vessels on the optic disc, retina, iris and angle structures (See Figure 2). Retinal ischemia resulting from progressive retinal capillary closure stimulates the release of angiogenic factors, such as vascular endothelial growth factor and placental growth factor. Such molecular mediators play an important role in promoting neovascularization and fibrous tissue proliferation. The new blood vessels that form are fragile and bleed easily when subject to vitreous traction, resulting in vitreous, preretinal and retinal hemorrhages. When the fibrovascular proliferation regresses it leaves behind a fibrous tissue that is attached to both the retina and the posterior hyaloid. Such fibrous tissues allow traction to be transmitted to the retina during vitreous contraction, resulting in tractional retinal detachment (TRD) and retinal break.
The first-line treatment for diabetic retinopathy is prevention. Tight blood glucose and blood pressure control have been shown to slow the onset and progression of DR. The Diabetes Control and Complications Trial (DCCT), conducted in North America, followed 1,141 subjects with type I diabetes for 10 years. The study showed that maintaining tight blood glucose can slow the onset and progression of DR.3 The United Kingdom Prospective Diabetes Study (UKPDS) recruited 5,102 patients with newly diagnosed type II diabetes and evaluated whether intensive control of blood glucose and blood pressure (for those who were also hypertensive) would reduce microvascular complications. UKPDS showed that blood glucose and blood pressure control can prevent and delay the progression of diabetic retinopathy in patients with type II diabetes.4,5
Patients with progressive DR may require intervention. Pan-retinal photocoagulation has been shown to decrease the risk of severe vision loss in patients with PDR. The Diabetic Retinopathy Study (DRS) was a randomized, controlled study that investigated the benefit of PRP in more than 1,700 patients with high-risk PDR (HR-PDR). The study revealed that PRP decreased the risk of severe visual loss (defined as visual acuity <5/200) by 50 percent when compared to observation.6
Another randomized control study, the Early Treatment Diabetic Retinopathy Study, investigated the benefit of PRP in more than 3,700 patients with early PDR or severe NPDR. The study revealed that PRP decreases the risk of progression to HR-PDR by 50 percent.7 These studies provided the justification for treating PDR with panretinal laser photocoagulation.
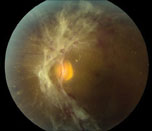 |
| Figure 3. Color fundus photo of the left eye shows tractional retinal detachment involving the macula. |
The use of preoperative anti-VEGF agents in diabetic vitrectomy has been shown to reduce intraoperative hemorrhage, decrease surgical time and make the manipulation of fibrovascular tissues easier.10-13 The regression of neovascular tissues following injection of an anti-VEGF agent likely accounts for these reported benefits. The main concern with using an anti-VEGF agent preoperatively is the possibility of exacerbating vitreoretinal tractions which may worsen the TRD or result in a retinal break, thereby creating a combined tractional-rhegmatogenous retinal detachment.14 This risk can be minimized by decreasing the duration between the injection of the anti-VEGF agent and the surgery. Studies have shown seven days duration between injection and vitrectomy to be safe.14
Diabetic Macular Edema
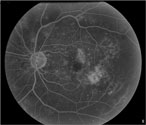 |
| Figure 4a. Fluorescein angiogram shows leakage from the perifoveal capillaries. |
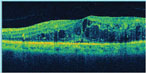 |
| Figure 4b. Optical coherence tomography shows macular edema. |
However, some patients are unresponsive or show less than ideal response to focal laser treatment and continue to lose vision despite extensive laser treatment. Thus, there is a great need for new mono or adjunctive therapies for DME. Some of the potential treatments for DME that have been studied are intravitreal corticosteroids, anti-vascular endothelial growth factor agents and surgical procedures.
1. Intravitreal Corticosteroids
Corticosteroid drugs have been demonstrated to inhibit the expression of both vascular endothelial growth factor gene and other anti-inflammatory mediators (such as prostaglandins). Triamcinolone, fluocinolone and dexamethasone are the main steroids that have been studied for the treatment of macular edema. The Diabetic Retinopathy Clinical Research Network (DRCR Net) was a multicenter randomized clinical trial that compared intravitreal triamcinolone acetonide as a monotherapy with focal/grid photocoagulation for the treatment of DME. The study randomized 840 eyes with DME to 1 mg of IVTA vs. 4 mg of IVTA vs. focal/grid laser. The 4-mg IVTA group had better visual acuity at four months; however, at 16 months, two years and three years, the laser group had better visual acuity than either IVTA groups. In addition, the laser group had fewer incidences of cataract and glaucoma.16,17
| Corticosteroid drugs have been demonstrated to inhibit the expression of both vascular endothelial growth factor gene and other anti-inflammatory mediators (such as prostaglandins). |
Ozurdex (dexamethasone intravitreal implant) is one such sustained-release formulation of corticosteroid that is injected intravitreally. Ozurdex has been approved by the FDA for the treatment of macular edema from retinal vein occlusion. Studies have also shown the benefit of Ozurdex in treating diabetic macular edema. A subgroup analysis of 171 eyes with persistent DME of ≥90 days that were treated with either 0.7 mg or 0.35 mg of Ozurdex showed better visual acuity (improvement of 10 letters or more), decreased central foveal thickness (CFT) and decreased leakage on fluorescein angiogram at 90 days compared to observation. However, at 180 days, no difference was found between the treatment and observation groups. Both the 0.7-mg and 0.35-mg groups had IOP elevation, but the rate was lower than what was reported for IVTA.18 A prospective study that evaluated the efficacy of 0.7 mg of Ozurdex for treatment-resistant DME in 55 vitrectomized eyes showed improvement of visual acuity and reduction of CFT at 26 weeks compared to baseline.18
A non-biodegradable ophthalmic insert of fluocinolone acetonide (Retisert) is FDA-approved for the treatment of non-infectious uveitis and is designed to release 0.59 mg/day of drug for about two-and-a-half years. A randomized trial comparing FA implant against standard of care (repeat laser or observation) in patients with DME demonstrated no evidence of edema in 58 percent of eyes receiving the implant versus 30 percent of eyes receiving standard of care, and a three or more line improvement in visual acuity was seen more often in test eyes (28 percent vs. 15 percent, p<0.05). However, in the device-implanted eyes, 95 percent of phakic eyes required cataract extraction, 35 percent had a rise in IOP, and 28 percent required a filtering procedure. (Pearson P, et al. IOVS 2006;47: E-Abstract 5442.)
Another fluocinolone acetonide implant, which is biodegradable, has been developed by Alimera Sciences, and marketed as Iluvien. Iluvien releases FA at a rate of either 0.2 or 0.5 µg per day for up to three years. Iluvien is delivered in to the eye with a 25-ga. needle, whereas Retisert is surgically implanted.
The Fluocinolone Acetonide for Macular Edema (FAME) studies conducted over a 36-month period included a total of 956 patients with DME. The study consisted of two separate prospective, randomized, controlled, double-masked, multicenter clinical trials conducted to assess the efficacy and safety of low dose (releasing 0.2 μg/day) and high dose (0.5 μg/day) intravitreal FA (Illuvien) in patients with DME. At 24 months, 28.7 percent of the low-dose and 28.6 percent of the high-dose group had 15 or more letter improvement, compared to 16.2 percent in the sham group (p=0.002 for both). Preliminary analysis at 30 months shows a 15 or greater letter improvement of visual acuity in 39.8 percent of patients receiving the low-dose insert. A report of 36-month data presented at the Association for Research in Vision and Ophthalmology 2011 meeting showed that a subgroup of patients with DME of greater than three years duration appears to have a greater benefit-to-risk ratio than those with DME less than three years of duration. At 24 months, 3.7 percent of the low-dose, 7.6 percent of the high-dose, and 0.5 percent of the sham groups required incisional glaucoma surgery. Cataract developed more frequently in the treatment group, with about 75 percent of the initially phakic patients undergoing cataract surgery at 24 months.20
2. Anti-VEGF Therapies
While steroids may play a role in refractory DME, the complications associated with the treatment have lead to interest in anti-VEGF agents for the treatment of DME. Ranibizumab (Lucentis), a recombinant humanized monoclonal antibody fragment, was specifically designed for ophthalmic use, and has been approved by the FDA for the treatment of neovascular AMD and retinal vein occlusion.
The Ranibizumab for Edema of the Macula in Diabetes (READ-2) study is a prospective, randomized clinical trial which compared ranibizumab alone vs. laser alone vs. a combination of ranibizumab and laser for the treatment of DME in 126 patients. At six months, treatment with ranibizumab resulted in a significantly better visual outcome than focal/grid laser treatment. The combined treatment showed no significant advantage over the other two groups.21 The study was continued for 24 months and concluded that intravitreal ranibizumab provided benefit for patients with DME for at least two years, and when combined with focal or grid laser treatments, the amount of residual edema was reduced, as were the frequency of injections needed to control edema.22
A DRCR study included 854 eyes with DME and compared intravitreal ranibizumab with prompt or deferred laser to IVTA with laser or laser with sham injections. At 12 months, both the ranibizumab/prompt laser and ranibizumab/deferred laser groups had significantly greater improvement in visual acuity from baseline compared to the IVTA/laser or sham/laser groups. Fifteen or more letters gain from baseline was seen in 28 to 30 percent of the ranibizumab groups versus 15 percent for the sham/laser group. In addition, rates of moderate vision loss (three lines) were lower in the ranibizumab groups compared to the laser group.23 The expanded two-year results were similar to results published at one year and reinforce the conclusions originally reported.24
Consistent with the results of the DRCR study, two Novartis-sponsored clinical trials, RESOLVE and RESTORE, showed that ranibizumab was superior in providing rapid and sustained visual acuity gain when compared to sham or laser therapy.25,26
Bevacizumab (Avastin), a full-length anti-VEGF antibody, was originally formulated for intravenous administration in the treatment of colon cancer. However, because of its reduced cost and similar effects when compared with ranibizumab it has been studied in multiple clinical trials. The DRCR study compared focal photocoagulation and intravitreal injection of bevacizumab for the treatment of DME. The data showed that central subfield thickness (CST)reduction was present at three weeks in 36/84 (43 percent) bevacizumab-treated eyes and in 5/18 (28 percent) eyes treated with laser alone, and at six weeks in 31/84 (37 percent) and 9/18 (50 percent) eyes, respectively. Combining focal photocoagulation with bevacizumab resulted in no apparent short-term benefit or adverse outcomes.27
Another trial, the two-year BOLT study, supported the use of intravitreal bevacizumab in CSME. Eighty patients were randomized to receive either bevacizumab or laser therapy. The bevacizumab group gained a median of eight ETDRS letters over 12 months, compared with a loss of 0.5 letters in the laser group.28 These studies demonstrate a role for bevacizumab in DME, and its reduced cost when compared to ranibizumab makes it more appealing. Future studies comparing the efficacy and safety of ranibizumab and bevacizumab for the treatment of DME should yield important information.
3. Surgical Management
Pars plana vitrectomy has been demonstrated to improve vision and reduce macular edema. Surgery is performed when DME persists despite multiple laser treatments. In 2007, reseachers in New Delhi, India, prospectively randomized 24 eyes of 24 patients with metabolically stable diabetes and with diffuse DME to either PPV with removal of internal limiting membrane (n=12 eyes) or modified grid laser photocoagulation (n=12 eyes). There was no statistical difference in visual acuity between the two groups at the end of six months; however, foveal thickness and macular volume decreased significantly more in the ILM group compared to the laser group.29 A larger prospective cohort study by the DRCR group was designed to investigate vitrectomy as a treatment for DME in 87 eyes with at least moderate vision loss and vitreomacular traction. At six months 43 percent of the eyes had CST <250 µm and 68 percent had at least a 50-percent reduction in thickening. Visual acuity improved by 10 or more letters in 38 percent and deteriorated by 10 or more letters in 22 percent. The study continued for one year, but little change in results was noted between six months and one year.30
Ongoing Studies
The 24-month results of the two pivotal Phase III trials (RISE and RIDE) were recently released by Genentech. The studies evaluated the safety and efficacy of ranibizumab in patients with DME. The data showed that patients who received ranibizumab had significantly more rapid and sustained improvement of vision compared to patients who received sham injection.31,32
VEGF Trap-Eye is an anti-VEGF compound that is designed to degrade more slowly in the eye. As a result, it may have a longer duration of action and reduce the frequency of intravitreal injection. In addition, VEGF Trap blocks not only the actions of VEGF but also the actions of placental growth factors, which are not blocked by the other anti-VEGF medications. The DaVinci study is comparing VEGF Trap-Eye with macular laser for the treatment of DME. Preliminary results show that at 24 weeks the patients in the VEGF Trap group showed significant gains in visual acuity, and the gains were sustained for a year.33
In conclusion, PDR and DME can lead to severe vision loss. The vision- threatening complications of PDR, tractional retinal detachment and vitreous hemorrhage can be prevented by timely PRP. New pharmacotherapies for the treatment of DME are showing good results. Anti-VEGF agents are effective, with good safety profile, but their short duration of action requires frequent injections. Sustained-release steroid delivery systems have addressed the need for less frequent injections; however the risk of cataract formation and IOP elevation remains a concern with intravitreal steroid use. Until we have a safe and longer-acting treatment for DME, combination therapy utilizing currently available pharmacotherapies with macular laser appears to be a more practical and sustainable approach.
The authors are on the Vitreoretinal Service in the Department of Ophthalmology at Wayne State University School of Medicine, Kresge Eye Institute. None of the authors has any financial interest in any product mentioned. Contact Dr. Tewari at the Kresge Eye Institute, 4717 St. Antoine Blvd., Detroit, Mich., 48201. Phone: (313) 993-0871, fax: (313) 577-2905, e-mail:
atewari@med.wayne.edu.
1. Fong DS, Aiello L, Gardner TW, King GL, et al. Retinopathy in diabetes. Diabetes Care 2004;27(Suppl 1): S84-S87.
2. Saaddine JB, Honeycutt AA, Narayan KMV, Zhang X, Klein R, Boyle JP. Projection of Diabetic Retinopathy and Other Major Eye Diseases Among People With Diabetes Mellitus: United States, 2005-2050. Arch Ophthalmol 2008;126:1740-1747.
3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-986.
4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853.
5. UK Prospective Diabetes Study (UKPDS) Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus (UKPDS 69). Arch Ophthalmol 2004;122:1631-1640.
6. The Diabetic Retinopathy Study Research Group. Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14. Int Ophthalmol Clin 1987;27:239-253.
7. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796-806.
8. Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study Report 2. Arch Ophthalmol 1985;103:1644-1652.
9. Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study Report 5. Arch Ophthalmol 1990;108:958-964.
10. da R Lucena D, Ribeiro JA, Costa RA, Barbosa JC, et al. Intraoperative bleeding during vitrectomy for diabetic tractional retinal detachment with versus without preoperative intravitreal bevacizumab (IBeTra study). Br J Ophthalmol 2009;93:688-691.
11. Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, Tano Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009;116:927-938.
12. Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefe’s Arch Clin Exp Ophthalmol 2008;246:837-842.
13. Yeoh J, Williams C, Allen P, Buttery R, Chiu D, Clark B, Essex R, McCombe M, Qureshi S, Campbell WG. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: A prospective case series. Clin Experiment Ophthalmol 2008;36:449-454.
14. Arevalo JF, Maia M, Flynn HW Jr. et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008;92: 213-216.
15. di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 2010;248(6):785-91.
16. Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008;115:1447-9.e1-10.
17. Diabetic Retinopathy Clinical Research Network. Three-year follow up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009;127:245-51.
18. Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, Whitcup SM. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Dexamethasone DDS Phase II Study Group. Arch Ophthalmol 2010;128:289-96.
19. Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li XY, Liu CC, Lou J, Whitcup SM. Dexamethasone Intravitreal Implant for Treatment of Diabetic Macular Edema in Vitrectomized Patients. For the Ozurdex CHAMPLAIN Study Group. Retina 2011;31:915-923.
20. Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, Gonder J, Kapik B, Billman K, Kane FE; FAME Study Group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118:626-635.e2.
21. Nguyen QD, Shah SM, Khwaja AA et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009;116:2175-81.
22. Nguyen QD, Shah SM, Khwaja AA et al. Two-year outcomes of ranibizumab for edema of the macular in diabetes (READ-2) study. Ophthalmology 2010;117:2146-51.
23. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064-1077.e35
24. Diabetic Retinopathy Clinical Research Network. Expanded 2-year Follow-up of Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2011;118:609-14.
25. Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010;33(11):2399-405.
26. Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A; RESTORE study group. The RESTORE study: Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615-625.
27. Diabetic Retinopathy Clinical Research Network (2007) A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114:1860-7.
28. Michaelides M, Kaines A, Hamilton RD et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology 2010;117:1078-1086.
29. Kumar A, Sinha S, Azad R, Sharma YR, Vohra R. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefe’s Arch Clin Exp Ophthalmol 2007;Mar;245(3):360-8. Epub 2006 Nov 9.
30. Diabetic Retinopathy Clinical Research Network Writing Committee on behalf of the DRCR.net, Haller JA, Qin H, Apte RS, Beck RW, Bressler NM, Browning DJ, Danis RP, Glassman AR, Googe JM, Kollman C, Lauer AK, Peters MA, Stockman ME. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 2010;117:1087-1093.e3.
31. Clinical trials.gov. A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus (RISE). Available from: http://clinicaltrials.gov/ct2/ show/NCT00473330 [Last accessed 27 September 2010]
32. Clinical trials.gov. A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus (RIDE). Available from: http://clinicaltrials.gov/ct2/ show/NCT00473382 [Last accesssed 27 September 2010]
33. The DA VINCI Study: Phase 2 Primary Results of VEGF Trap-Eye in Patients with Diabetic Macular Edema. Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Rückert R, Sandbrink R, Stein D, Yang K, Beckmann K, Heier JS. Ophthalmology 2011;118:1819-26.