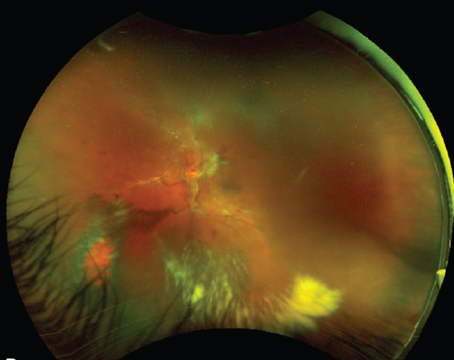
Presentation
A 63-year-old woman presents with floaters and hazy vision in the left eye for the past week.
History
Her past ocular history was significant for a golf ball injury to the right eye five months prior to presentation. Her past medical history included breast cancer treated with complete resection via mastectomy 10 years prior to presentation, hypercholesterolemia, hypertension and gastroesophageal reflux disease. She received the Pfizer-BioNTech Bivalent COVID-19 vaccine one week before symptom onset.
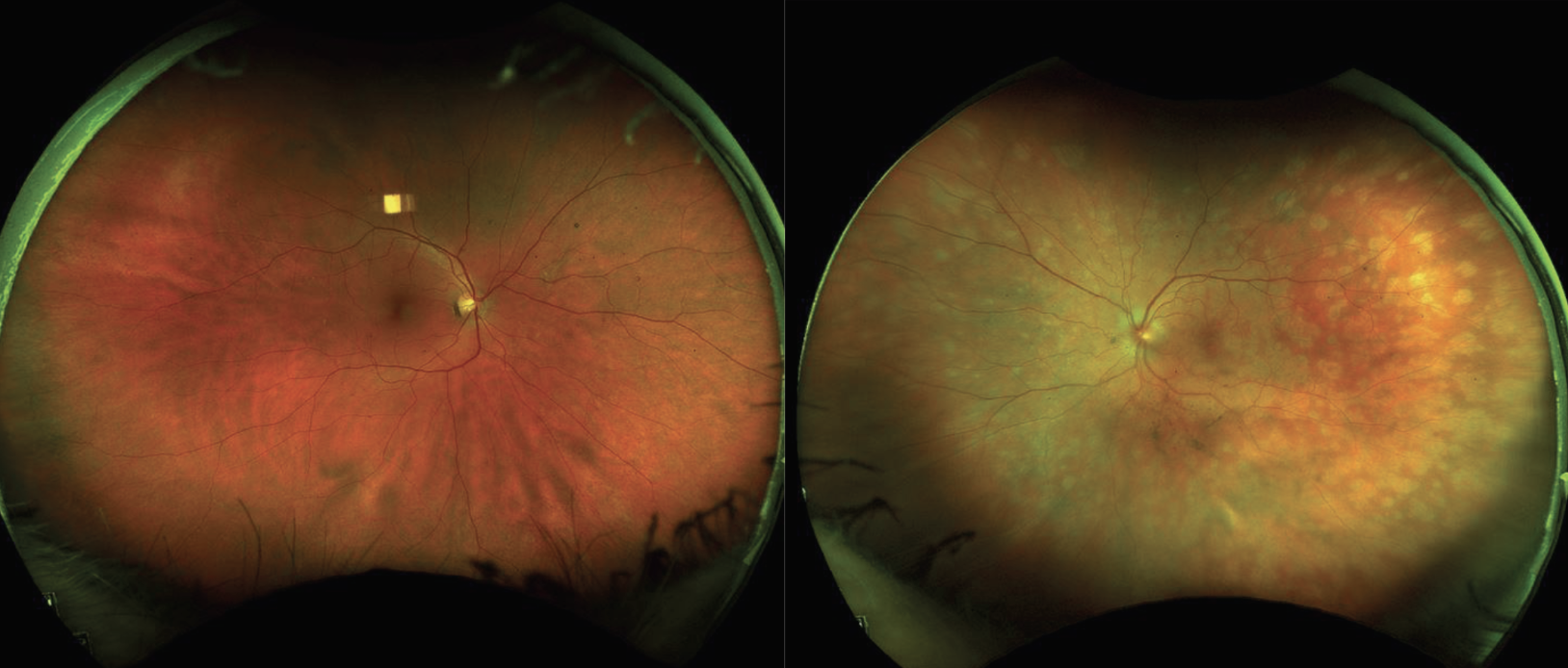 |
| Figure 1. Ultra-widefield fundus photographs of both eyes at presentation. The right eye was unremarkable except for a small choroidal nevus in the superior mid-periphery. The left eye showed multifocal, coalescing white spots that extended from the posterior pole to the periphery with associated optic nerve edema. |
Examination
On presentation, visual acuity was 20/20 OD and 20/80 OS. Intraocular pressure was 14 mmHg in both eyes and there was no relative afferent pupillary defect. Extraocular motility was full, and the patient was able to see 8 out of 8 Ishihara color plates OU. The anterior segment exam was unremarkable OD and revealed 1+ anterior chamber cell and trace anterior vitreous cell OS. The fundus exam OD was only notable for a small choroidal nevus in the superior mid-periphery. Funduscopic examination OS showed multifocal, coalescing white spots that extended from the posterior pole to the periphery with associated optic nerve edema (Figure 1).
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
Ancillary imaging was obtained. Fundus autofluorescence OD was unremarkable and OS revealed hyper-autofluorescence corresponding to the white retinal lesions seen on examination OS (Figure 2). Optical coherence tomography OD was unremarkable, and OS revealed segmental disruption and loss of the ellipsoid zone (Figure 3). Fluorescein angiography OD was unremarkable, and OS revealed early punctate hyperfluorescence distributed in a wreath-like configuration with late staining of the lesions and the optic nerve (Figure 4).
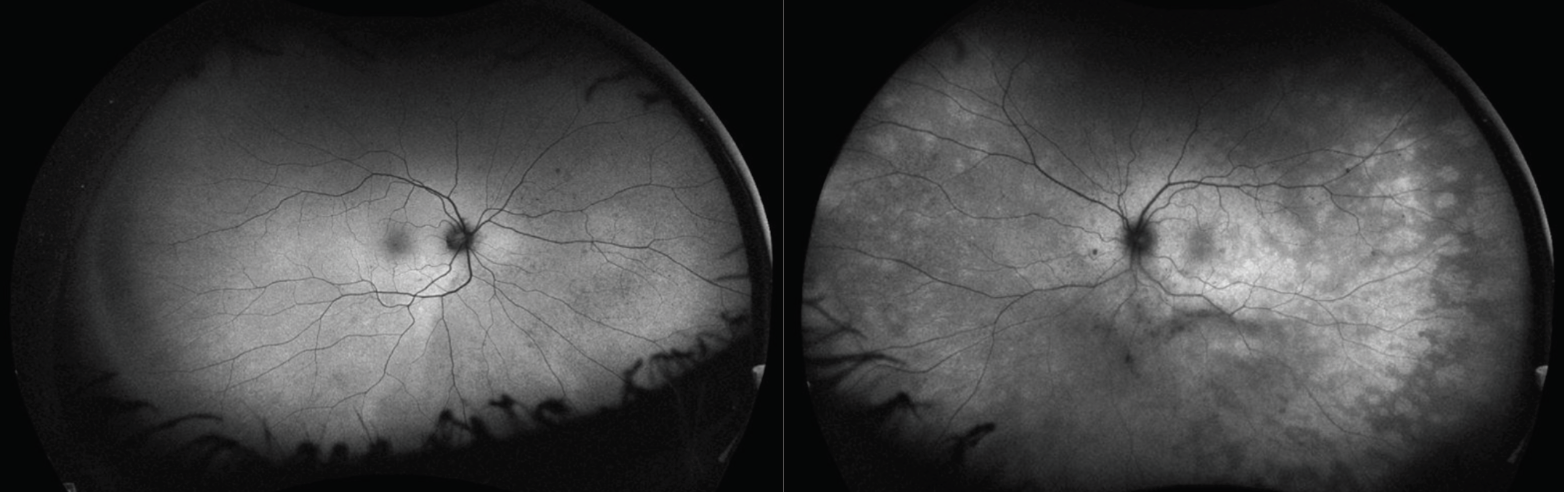 |
| Figure 2. Ultra-widefield fundus autofluorescence of the right eye was unremarkable, and the left eye revealed hyperautofluorescence corresponding to the white retinal lesions seen on examination. |
The differential diagnosis for this case broadly included inflammatory, infectious and malignant etiologies. Of the inflammatory etiologies, multiple evanescent white dot syndrome (MEWDS) was considered the most likely; other less likely conditions included acute posterior multifocal placoid pigment epitheliopathy (APMPPE), multifocal choroiditis, posterior inner choroidopathy and sarcoidosis. Infectious etiologies included syphilis and tuberculosis.
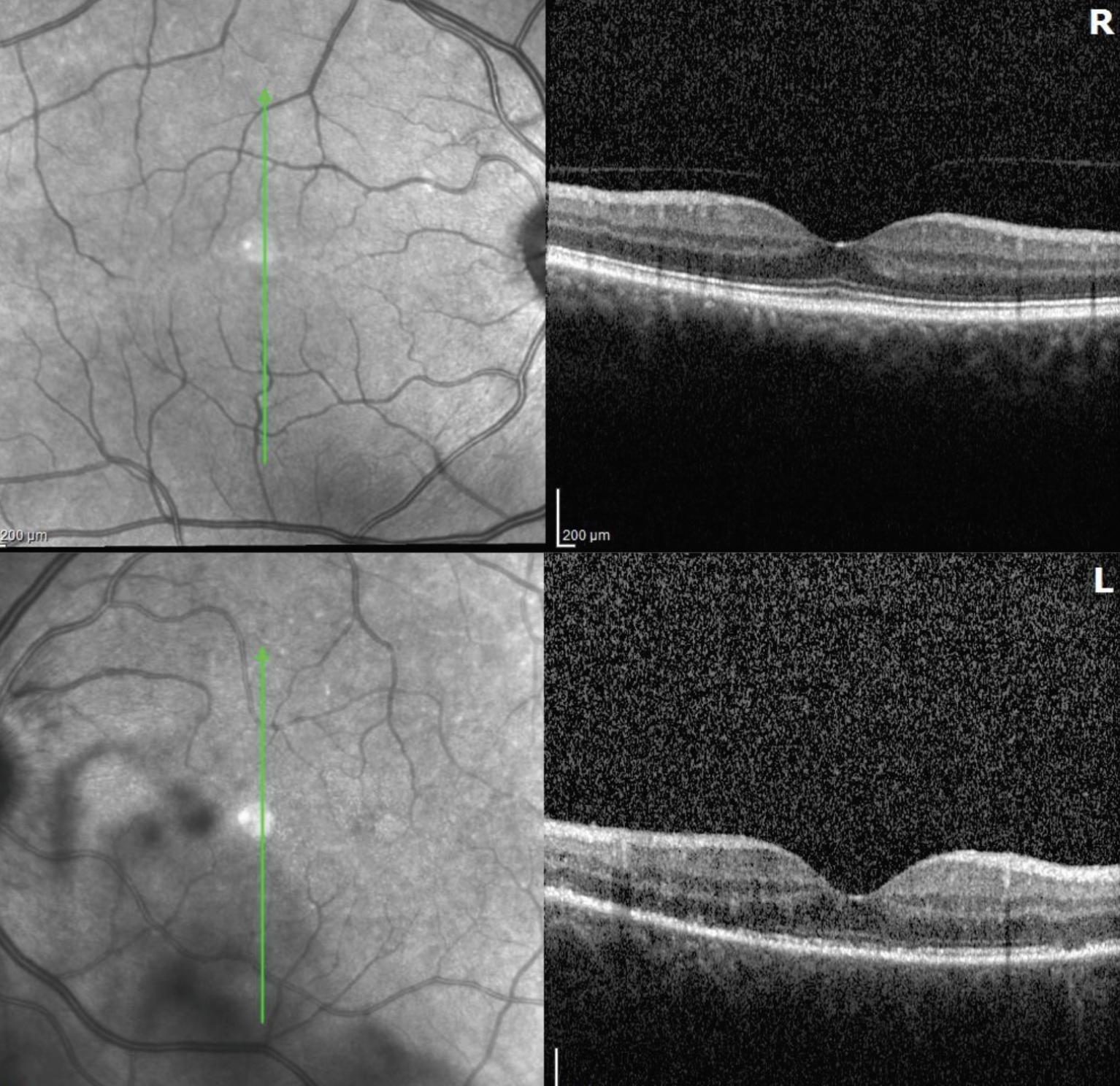 |
| Figure 3. Optical coherence tomography of the right eye was unremarkable while the left eye revealed multifocal segmental loss of the ellipsoid zone. |
Although less likely, vitreoretinal lymphoma should remain on the differential diagnosis.
The patient was asked to get a laboratory work-up, including a complete blood count with differential, syphilis antibody testing, QuantiFERON gold, angiotensin converting enzyme and chest X-ray. All testing came back unremarkable.
Given the temporal relationship with her immunization, a suspected diagnosis of a MEWDS-like reaction secondary to the Pfizer-BioNTech Bivalent COVID-19 vaccine was made. The risks and benefits of systemic corticosteroids were discussed with the patient. Given her atypical features including her age, emmetropia and temporal association with COVID-19 immunization, the decision was made to start her on 50 mg of prednisone once daily with a weekly taper by 10 mg. Topical prednisolone acetate 1.0% was started to treat her anterior chamber reaction.
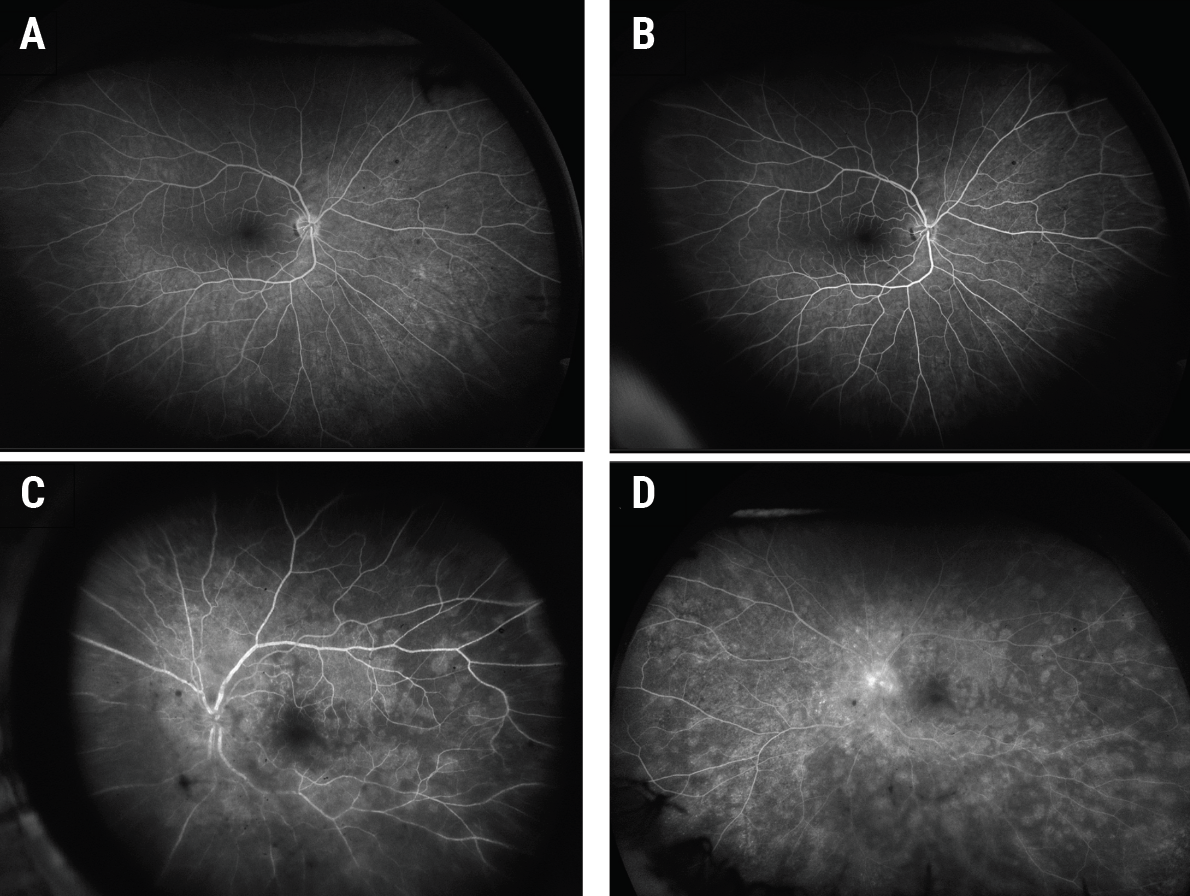 |
| Figure 4. Early (A) and late (B) fluorescein angiography (FA) of the right eye was unremarkable. Early FA of the left eye (C) showed early punctate hyperfluorescence of the white retinal lesions in a wreath-like configuration, while a late frame (D) showed increased staining of the lesions and the optic nerve. |
The patient followed up two weeks later with great improvement in symptoms. Her visual acuity in the affected left eye had improved to 20/40. There was resolution of intraocular inflammation within the anterior chamber and vitreous. There was a notable reduction in the number, size and the prominence of the retinal lesions on examination and FAF OS (Figure 5). At her most recent follow-up, six weeks from initial presentation, her acuity improved to 20/20 and there was near complete resolution of the retinal lesions on examination and FAF OS (Figure 6). OCT of the macula OS revealed further reconstitution of the ellipsoid zone (Figure 7).
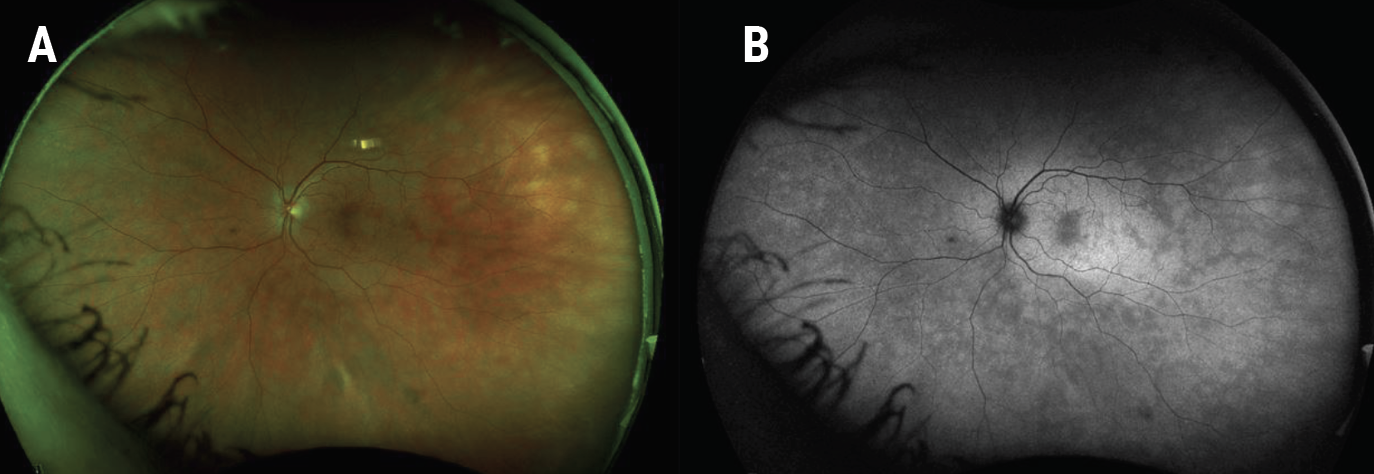 |
| Figure 5. Ultra-widefield fundus photograph of the left eye two weeks after presentation showed a reduction in the number, size and prominence of the white retinal lesions (A). Similarly on fundus autofluorescence there was an improvement in the number and size of the hyperautofluorescent lesions (B). |
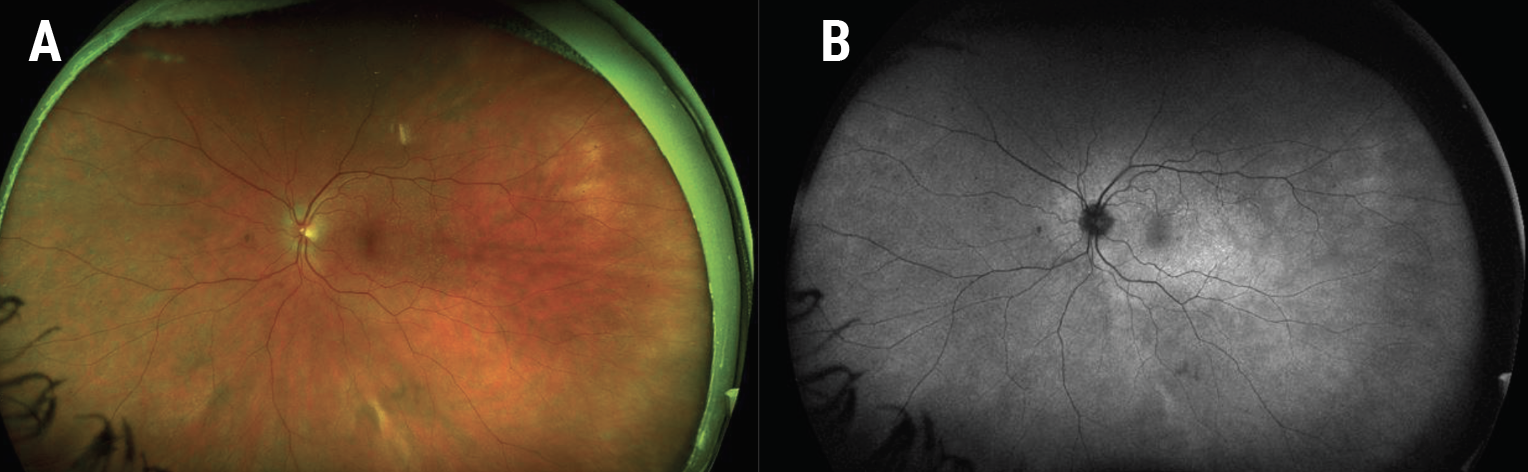 |
| Figure 6. Ultra-widefield fundus photograph of the left eye six weeks after presentation revealing near resolution of the white retinal lesions (A). Similarly, there was near resolution of the hyperautofluorescent lesions on fundus autofluorescence (B). |
Discussion
According to the recent publication by the Standardization of Uveitis Nomenclature working group, the classification criteria for MEWDS should include: 1) multifocal gray-white chorioretinal spots with foveal granularity; 2) characteristic wreath-like hyperfluorescent lesions on fluorescein angiography and/or outer retinal hyperreflective lesions on OCT; and 3) absent to mild anterior chamber and vitreous inflammation.1 Classically, MEWDS is a predominantly unilateral disease which occurs mostly in young to middle-aged (mean 35.2 years) myopic (mean -1.6 D) females following a viral prodrome.2 The condition is typically self-limited with spontaneous recovery. Approximately 95 percent of all eyes diagnosed with MEWDS achieve a VA of 20/25 or better upon disease resolution.3
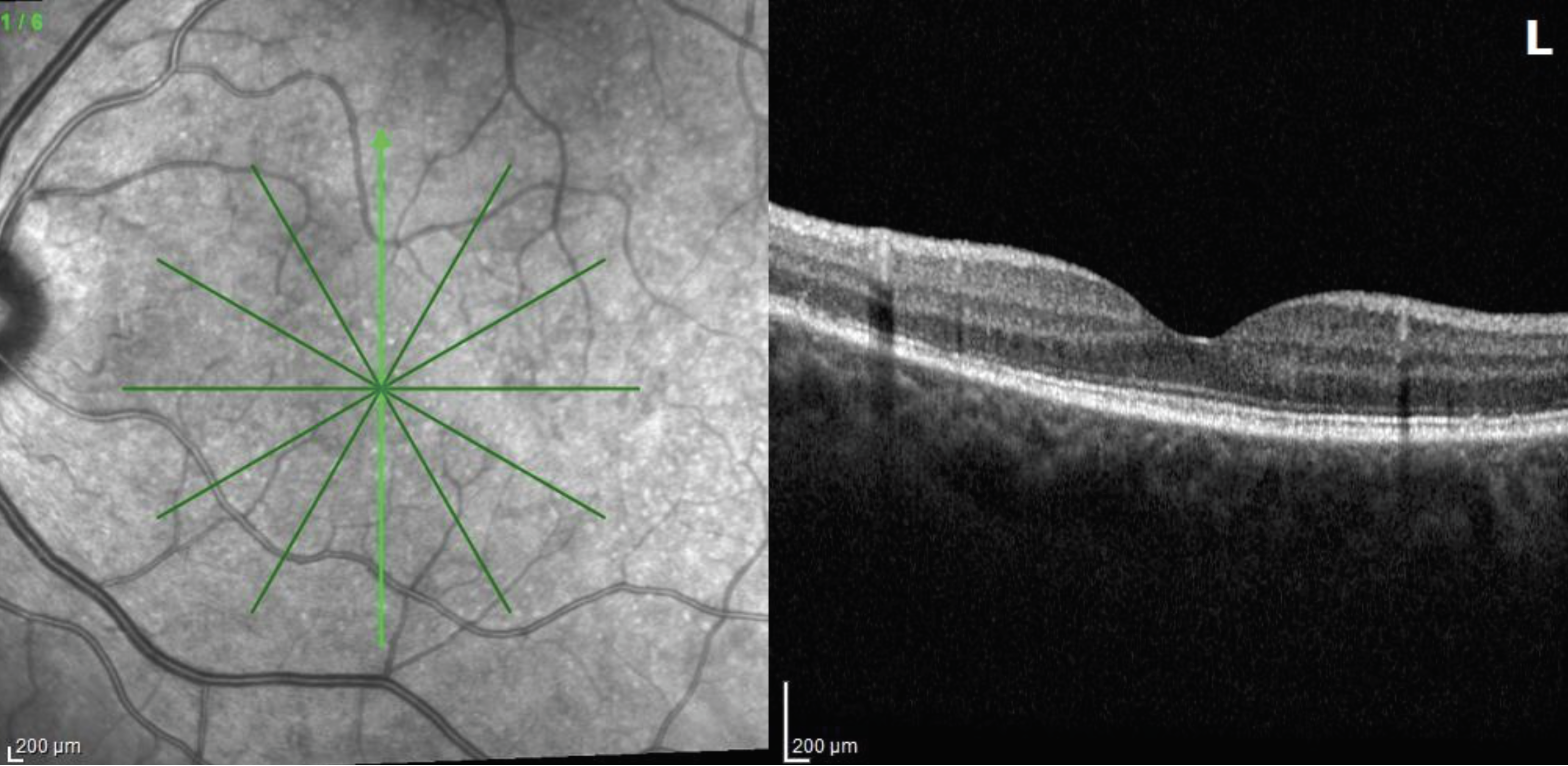 |
| Figure 7. Optical coherence tomography of the left eye six weeks after presentation revealing further reconstitution of the ellipsoid zone. |
Interestingly, our patient was a 61-year-old emmetropic female without a viral prodrome. We suspect that this is a MEWDS-like reaction to the Pfizer-BioNTech Bivalent COVID-19 vaccine. Numerous uveitic adverse events have been documented post-COVID-19 vaccination, suggesting a possible causal relationship.4,5 However, given the sheer number of individuals being vaccinated against COVID-19 this relationship is very difficult, if not impossible, to determine with confidence. The reported uveitic manifestations after COVID-19 vaccination are varied and include scleritis, anterior, intermediate, posterior, and panuveitis along with retinal vasculitis, acute macular neuroretinitis, MEWDS, Vogt-Koyanagi-Harada syndrome and Behçet’s disease.4,5 It’s been noted that these adverse ocular events seem to mirror those that can occur in the setting of a COVID-19 infection, suggesting a possible common pathway between the virus and vaccine-mediated ocular immune response.4 Similar to our case, one study described two cases of a MEWDS-like reaction after the second dose of the BNT162b2 mRNA vaccination, commonly recognized as the original Pfizer vaccine. In the cases described, the mean time from vaccination to MEWDS onset was 7.5 days. Both of these patients had complete spontaneous resolution of all symptoms.6
Because our patient was atypical with a rather aggressive presentation, we discussed the risk and benefits and started her on oral prednisone. Thankfully, she had a rapid improvement in her symptoms and retinal lesions with a return of VA back to 20/20 OS.
Finally, we must consider the role of additional vaccinations against COVID-19 in this patient. The risk of future ophthalmic side effects must be weighed against the risk of contracting severe COVID-19 infection. Additionally, there’s a concern that direct infection with COVID-19 may also be associated with de novo uveitis and reactivating previously quiescent uveitis. We’ve asked the patient to refrain from getting additional COVID-19 vaccinations at this point and to take all precautions against exposure to the COVID-19 virus. This should be a continued conversation between the patient and provider over the entirety of their relationship, as the balance of risks and benefits can change with time.
1. Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for multiple evanescent white dot syndrome. Am J Ophthalmol 2021;228:198-204.
2. Ramakrishnan MS, Patel AP, Melles R, Vora RA. Multiple Evanescent white dot syndrome: Findings from a large Northern California cohort. Ophthalmol Retina 2021;5:9:850-854.
3. Marsiglia M, Gallego-Pinazo R, Cunha de Souza E, et al. Expanded clinical spectrum of multiple evanescent white dot syndrome with multimodal imaging. Retina (Philadelphia, Pa) 2016;36:1:64-74.
4. Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm 2021;29:6:1216-1224.
5. Bolletta E, Iannetta D, Mastrofilippo V, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med 2021;10:24.
6. Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, et al. Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: A possible association. Retina (Philadelphia, Pa) 2021;41:12:2462-2471.