Ophthalmic ultrasound has evolved from its introduction into the clinical setting in 19521 to the current A- and B-scan contact and immersion techniques which are widely used for the characterization of intraocular and orbital pathology.1 It currently stands as the principal diagnostic tool for a number of conditions within the eye and as a supplement to the radiologic imaging capabilities of MRI and CT scanning within the orbit.
Ophthalmic ultrasound has evolved from its introduction into the clinical setting in 19521 to the current A- and B-scan contact and immersion techniques which are widely used for the characterization of intraocular and orbital pathology.1 It currently stands as the principal diagnostic tool for a number of conditions within the eye and as a supplement to the radiologic imaging capabilities of MRI and CT scanning within the orbit.
Fundamental Principles
The fundamental physical principle underlying diagnostic ultrasound is the generation of sound waves at frequencies above the range of human hearing (greater than 20,000 Hz or 20 KHz) by the vibration of a thin crystal in the tip of the probe which is stimulated by pulses of electric current. The waves of sound then propagate through a medium and are reflected by tissue interfaces back to the resting crystal that is then made to vibrate, generating electrical impulses that are amplified and processed to show a pattern on an oscilloscope screen. The B-scan (brightness amplitude) probe contains a transducer which sweeps back and forth at an average rate of 25 oscillations per second.
 |
Since the early ’80s, the most common indication for intraocular echography continues to be for the measurement of the ocular axial length as an essential element in the calculation of intraocular lens power. Recently the predominance of echography has been challenged by partial coherence interferometry as applied in the IOLMaster. However, there are a number of instances of media opacities where this light-based technology can’t be used, including corneal edema, corneal scarring, dense nuclear and certain posterior subcapular cataracts, and some vitreous opacities. One study found that about 20 percent of cataract patients couldn’t be measured by the IOLMaster and required immersion ultrasound.3
 B-scan is less useful than the A-scan in ocular biometry with the exception of macular staphylomas in highly myopic eyes. In these cases an immersion 10-MHz B-scan displays the cornea and the staphyloma in the same image. A vector A-scan is then superimposed on the B-scan and aligned with the center of the staphyloma to allow an accurate axial length measurement.4
B-scan is less useful than the A-scan in ocular biometry with the exception of macular staphylomas in highly myopic eyes. In these cases an immersion 10-MHz B-scan displays the cornea and the staphyloma in the same image. A vector A-scan is then superimposed on the B-scan and aligned with the center of the staphyloma to allow an accurate axial length measurement.4 Value in Detecting Pathology
Echography is essential in the evaluation of the internal globe in cases of media opacity. Pathological conditions such as retinal detachment and intraocular tumors are most accurately and cost-effectively diagnosed by ultrasound. There are a number of reports in the literature of unsuspected intraocular tumors that were not discovered until after cataract surgery. In one, researchers reviewed 21 cases of cataracts that had been removed in the presence of unsuspected choroidal or ciliary body melanomas. The authors stated, “… since ultrasonography has become readily accessible to most ophthalmologists in countries with advanced medical care, it should be considered as a part of the preoperative evaluation in all patients who have a cataract which is advanced enough to prevent a clear fundus view. It should definitely be performed in patients who have a dense unexplained unilateral cataract.”5
 |
 |
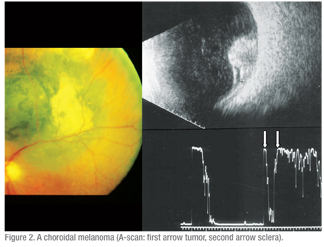
Echography is equally important in the evaluation of visible fundus lesions. A-scan provides the unique capacity to characterize the internal structure of intraocular tumors that is highly correlated to the tissue characteristics of the lesion. The quantitative (spike height and regularity) and kinetic (rapid spike movement) criteria provide high specificity and sensitivity in the evaluation of ocular lesions.9 (See Figures 1 & 2)
Even after the advent of widespread use of indirect ophthalmoscopy and fluorescein angiography in the evaluation of intraocular tumors, misdiagnosis was found to be as high as 20 percent.10 Since this study, echography has dramatically impacted the accuracy of the evaluation of intraocular tumors. This technique began to be used more extensively in the 1970s and the rate of specificity for the diagnosis of choroidal melanoma increased significantly. Data from the Collaborative Ocular Melanoma Study (COMS) documents a diagnostic accuracy of 99 percent, although atypical lesions were not included.11 Standardized echography was the primary ancillary tool used in this study.
 |
The A-scan provides unique information about orbital lesions beyond that supplied by topography-based studies such as B-scan and radiologic imaging. The American Academy of Ophthalmology’s Basic and Clinical Science Course recognizes that “standardized A-scan is much less aesthetically attractive to the beginner and is more difficult to perform. However, it potentially conveys much more diagnostic information than the B-scan.”12 It is useful in the quantitation of orbital structures, such as the extraocular muscles and the optic nerve, and the thickness of the lacrimal gland. It can also provide information about the paranasal sinuses, nasolacrimal system, posterior sclera and sub-Tenon’s space. It provides information about the internal structure of intraocular and orbital tumors that is often highly correlated to the pathological diagnosis.
Broad-Based Study Undertaken
A 2004 study addressed the impact of echography on the evaluation and management of posterior segment disorders. The group summarized the accuracy of the ultrasound diagnosis as compared to the final clinical or pathological findings in the context of the impact of these diagnoses on the management of the respective pathological conditions.13
As there are no recent studies addressing the utilization of ophthalmic echography in current clinical practice, I undertook a retrospective review at our center to evaluate a broad spectrum of intraocular and orbital pathology as evaluated by echography. This study presents the diagnostic findings of 1,000 consecutive patients referred to an ophthalmic echographic specialty practice over a 16-month period ending in January 2009.
 |
 |
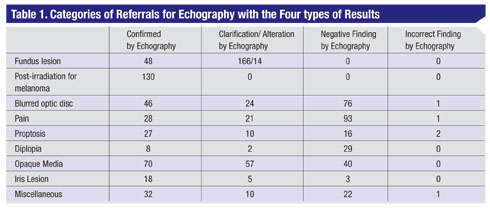
Ultrasound examinations were categorized into nine general areas (See Table 1). These nine categories were then subdivided into clinical results including: echographic confirmation of the referring doctor’s clinical diagnosis, clarification or alteration of the referred diagnosis by echographic findings, negative findings and incorrect echographic diagnosis. These patients were all examined by the same well-experienced, board-certified ophthalmologist who had completed a fellowship in ophthalmic ultrasound. The echographic equipment consisted of the Innovative Imaging V1 unit with a dedicated 10-mHz B-probe, a 20-mHz immersion B-probe and a separate 8-mHz diagnostic A-probe. In addition a 50-mHz UBM unit (Paradigm Medical Industries) was used for high-resolution imaging of the anterior segment. All patients were examined during the same session by both the A-scan and the B-scan except for those with anterior segment lesions (iris, lens and ciliary body) in whom the immersion technique was utilized with either a 20- or 50-mHz probe.
Patients’ average age was 51 years (r: 3 months to 91 years), with 465 males and 535 females.
Findings
Table 1 lists the specific findings by the nine categories. Some of the issues that related to each of these groups are:
• Fundus lesion. (n=228) Echography confirmed the referring clinician’s impression in 21 percent of cases. This included 23 with the diagnosis of choroidal or ciliary body melanoma and 24 referred as nevus. One hundred and sixty-six patients were referred only with the diagnosis of fundus lesion. In this group echography further clarified the diagnosis by specifying nevus in 114, and malignant melanoma in five. Fourteen patients were referred with a clinical impression that was altered by the echographic findings.
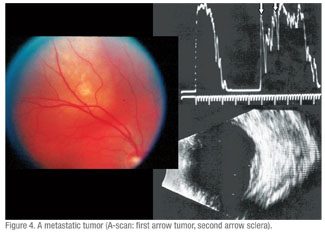
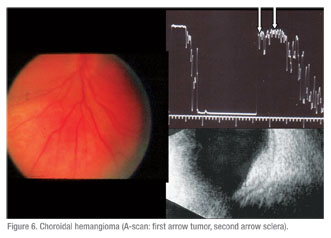
These included: five with dystrophic choroidal calcification (See Figure 3); one metastatic tumor (See Figure 4); two choroidal hemangiomas (See Figure 6); one retinoblastoma; one choroidal detachment; one retinal detachment; two subretinal disciform lesions; and one that was referred as melanoma but was not substantiated by echographic findings. This eye underwent a needle biopsy with non-specific findings that were not consistent with a melanoma.
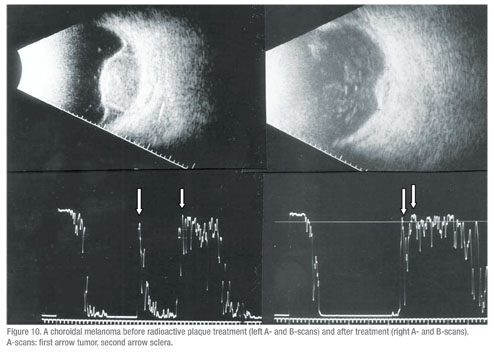 |
• Post radioactive iodine plaque treatment for choroidal melanoma.(n=130) All of the patients in this group showed a response to therapy with shrinkage of the tumor and concurrent A-scan changes (increased irregular internal reflectivity and reduced vascularity compared to pre-treatment A-scan findings (See Figure 10, p. 107). None of these eyes showed evidence of tumor growth in the 16 month time period of this study.
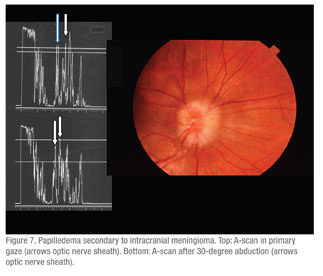
• Blurred discs with various diagnoses including calcified optic nerve head drusen, blurred optic nerve heads, or “to rule out papilledema.” (n=147) In this group 31 percent were confirmed as having calcified drusen, and in 52 percent no drusen or other abnormalities were found. In the eyes in which drusen were not found it was assumed that these were anamolous optic nerve heads based on the absence of other causes or based on clinical evidence and/or normal radiologic imaging studies. In the clarification/alteration group, echography documented the presence of calcified drusen in 13 percent of patients in whom they had not been expected clinically (See Figure 9). Three percent in this group had distended optic nerve sheaths consistent with increased intracranial pressure as quantified by A-scan measurements of a reduction in nerve sheath thickness by the 30-degree test (See Figure 7).14 In this test the optic nerve is measured by A-scan in primary gaze and then remeasured after the patient abducts the eye about 30 degrees. Three of these had confirmation of this on lumbar puncture with a high opening pressure. Two were felt to have idiopathic optic nerve sheath dilation. One patient in the incorrect echography group was a 13-year-old child who was diagnosed as having an optic nerve glioma by echography that later proved to be a meningioma on biopsy.
 |
• Proptosis. (n=55) Echography in 45 percent of these confirmed the clinical diagnosis as Graves’ disease, and in 4 percent as cases of cavernous hemangiomas as later verified by orbitotomy. Ten patients were found by echography to have diagnoses not noted by the referring clinician, including: seven patients with Graves’ disease; one with axial myopia; one with a mucocele; and one with orbital pseudotumor. Sixteen patients had no echographic basis for proptosis and no causes were found on subsequent clinical and/or radiographic evaluation.
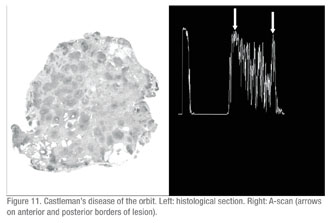
Two patients with proptosis were incorrectly diagnosed by echography. One was diagnosed as having a cavernous hemangioma which later proved to be a case of orbital Castleman’s disease on pathological evaluation. The follicular centers separated by sclerotic septae in this case were echographically similar to the multiple cavernous spaces in an orbital hemangioma (See Figure 11, p. 111). The other case was diagnosed as adenocystic carcinoma of the lacrimal gland and proved to be a pleomorphic adenoma (benign mixed cell tumor) on pathological examination.
• Diplopia. (n=39) The referring clinical diagnosis in 21 percent of patients was confirmed by echography. These patients were all diagnosed with Graves’. Two patients were found by ultrasound to have diagnoses that were not proposed by the referring clinician. One of these had a long history of myasthenia gravis with the recent onset of diplopia and echographic findings consistent with Graves’ disease. The other patient was highly myopic with a staphyloma, which was felt to be the basis for his diplopia. In the negative echographic findings group, 22 patients had no echographic cause for diplopia and subsequently underwent neurological evaluation and neuro-imaging, which was negative in 15 of these patients.
 |
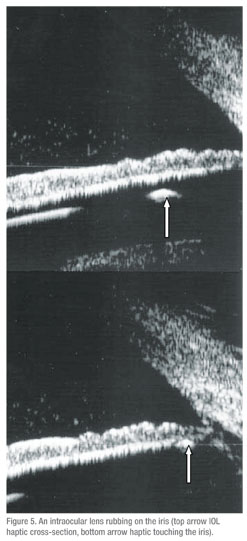
• Iris lesions. (n=26) Fifteen of these were confirmed as iris nevi and three as iris cysts. Five patients had echographic diagnoses that were unexpected by the referring clinician. Two of these were iris cysts, one was a foreign body, one was an iris melanoma, and one was an elevation of the temporal iris caused by a dislocated IOL haptic. Three patients referred with an iris elevation had no detectable lesion by echography.
• Miscellaneous. (n=67) Thirty-two of these cases had mostly subjective symptoms such as photopsias, transient double vision, eyestrain and transiently blurred vision. Echography confirmed the clinical impression in these cases. Ten of the patients had clarifying echographic findings such as shallow retinal detachment and staphyloma. Twenty-two patients in the miscellaneous category had no echographically detectable abnormalities. One of them was diagnosed by echography as having a lesion adjacent to the lacrimal gland, but lacrimal gland biopsy revealed low-grade dacryoadenitis and did not confirm another lesion.
Reducing ‘Defensive’ Medicine
Of 1,000 patients referred for ocular and/or orbital echography, the referring clinician’s impression was confirmed in 407 cases. No diagnostic echographic findings were present in 279 patients. Clinical impressions were clarified or altered by echography in 309 patients. Five patients were incorrectly diagnosed by echography.
The confirmation of the clinical diagnosis is important especially when invasive therapy is proposed such as radiation or enucleation in the case of choroidal or ciliary body malignant melanoma. Also, the echographic confirmation of the clinical impression of entities such as optic nerve head drusen can obviate the need for more extensive testing.15
The current Medicare allowable charge for diagnostic A- and B-scan with the CPT code 76510 is about $145.00.16 In comparison, radiologic studies can run into the thousands of dollars. Ideally the clinician’s conclusions based on a careful history and physical examination would suffice as the basis for therapeutic intervention or non-intervention in most cases. However, in our current medical system, there are strong pressures to practice defensive medicine by ordering diagnostic tests to support the clinical judgment. It has been estimated that such defensive medicine, including unnecessary testing, adds $1,700 to $2,000 per year to the average American family’s health-care costs.17 Patients with health insurance and their physicians are relatively insulated from the costs of these tests in the short term, but the impact is additive to the overall costs of U.S. health care.
Sixty-five of the 147 patients referred for evaluation of a blurred disc were found to have calcified optic nerve head drusen. Forty-six of these were suspected by the referring clinician, but 19 were unsuspected and underwent various degrees of evaluation from radiologic imaging studies including MRI and CT scan to neurological examinations and lumbar puncture. In patients with blurred discs in this study, 7.3 percent could have been spared the time and expense of further testing if echography was done as the primary imaging study.
Negative ultrasound findings occurred in 75 of the 147 patients with blurred discs or 51 percent. Most of these patients were ultimately diagnosed as having anomalous optic nerve heads on the basis of the subsequent clinical course or normal radiologic findings. It could be argued that echography added additional unnecessary expense in the evaluation of these patients, but given the greater costs of neuro-imaging studies ultrasound is a more cost-effective type of defensive medicine. The use of diagnostic A-scan in this group facilitated the evaluation of optic nerve sheath thickening, which is useful in identifying patients with true papil-ledema due to increased intracranial pressure.
The greatest percentage of negative ultrasound findings, 65 percent, occurred in those patients with the complaint of eye pain. Various types of pain are common symptoms in ophthalmic and optometric practices.18,19 This complaint requires a careful history and physical examination of the eye and paraocular structures, but in many instances the etiology cannot be determined. Echography is a reasonable ancillary test to consider in such cases because it is rapid, non-invasive and relatively inexpensive. Thirteen percent of these patients had unexpected findings detected by echography. Two of them had echographic findings unrelated to the symptom of pain (one shallow retinal detachment and one dystrophic choroidal calcification).
Limitations
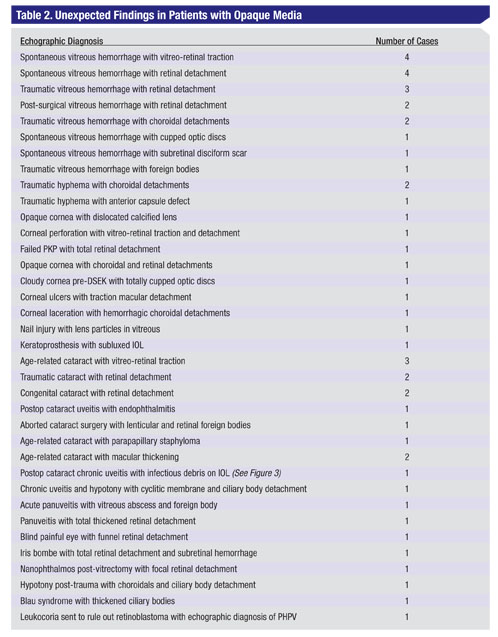
The highest percentage of unexpected findings (57 out of 144 patients or about 40 percent) occurred in the category of opaque media. The most common finding in this group was retinal detachment and/or vitreoretinal traction. This finding often resulted in surgical intervention, which is generally more effective when performed in a timely fashion (See Table 2).
The five patients incorrectly diagnosed by echography included one patient characterized as an optic nerve glioma, which later proved to be a meningioma. The second was a histologically proven pleomorphic adenoma of the lacrimal gland that was called an adenocystic carcinoma by echography. The third was a case of orbital Newcastle’s disease that was called a cavernous hemangioma by echography. The fourth was called a probable dacryoadenitis by ultrasound and proved to be a bony lesion on biopsy. The fifth was diagnosed as a lesion adjacent to the lacrimal gland by echography, but proved to be a low grade dacryoadenitis on biopsy.
The echographic examinations in this study were almost all performed with the use of both a B-scan probe and a separate A-scan probe for each patient examination. The ability to characterize the internal structure of intraocular and orbital lesions and to quantitate their thickness is heavily dependent on the standardized diagnostic A-scan, as developed by Karl C. Ossoinig, MD.20 Also, the measurement of orbital tissues such as the optic nerve, extraocular muscles and lacrimal glands is dependent on this modality.
The routine use of the diagnostic A-scan is limited to a relatively few centers in the United States so this study could be criticized because of its dependence on this modality. This analysis doesn’t seek to resolve that issue but simply presents the results of the combined use of modern ultrasound technology including B-scan, high frequency immersion B-scan, and diagnostic A-scan. Future comparisons would be necessary to address this question.
Ophthalmic echography as performed in this study was an important diagnostic tool in clinical practice. It provided essential information about the anterior and posterior segments in eyes with opaque media. It allowed for accurate diagnoses of intraocular and orbital conditions in which the results of clinical examinations and radiologic imaging studies were unclear. The results obtained in this review were heavily influenced by the use of the standardized A-scan.
Dr. Harrie is a clinical professor of ophthalmology at the Moran Eye Center, University of Utah, Salt Lake City and in private practice. Contact him at roger.harrie@hsc.utah.edu or (801) 535-8177.
1. Mundt GH Jr, Hughes WF Jr. Ultrasonics in ocular diagnosis. Am J Ophthalmol 1956;Mar;41(3):488-98.
2. Harrie RP. Clinical ophthalmic echography: A case study approach. New York: Springer: 2008.
3. Freeman G, Pesudovs K. The impact of cataract severity on measurement acquisition with the IOL master. Ophthalmologica Scandinavica 2005 Aug 83(4);439-442.
4. Zaldivar R, Shultz MC, Davidorf JM, et. al. Intraocular lens power calculations in patients with extreme myopia. J Cataract Refract Surg 2000;25:668-674.
5. Shields JA, Augsburger JJ. Cataract surgery and intraocular lenses in patients with unsuspected malignant melanoma of the ciliary body and choroid. Ophthalmology 1985;92:823.
6. Pe’er J, Savino DF, McLean IW, Zimmerman LE. Posterior uveal melanomas in aphakic and pseudophakic eyes. Am J Ophthalmol 1986;101:458–460.
7. Shammas HF, Blodi FC. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol 1977;95:63–69.
8. Rath S, Honavar SG, Naik MN, et al. Evisceration in unsuspected intraocular tumors. Arch Ophthalmol. 2010;128(3):372-379.
9. Ossoinig KC. Quantitative echography—The basis of tissue differentiation. J Clin Ultrasound 1974;2:33.
10. Shields JA, Zimmerman LE. Lesions simulating malignant melanoma of the posterior uvea. Arch Ophthalmol 1973:89:466-471.
11. Collaborative ocular melanoma study group. Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study. Arch Ophthalmol 2003:121:1163-1171.
12. American Academy of Ophthalmology basic and clinical science course Section 3. San Francisco: American Academy of Ophthalmology, 2005.
13. Scott IU, Smiddy WE, Feuer WJ, Ehlies FJ. The impact of echography on evaluation and management of posterior segment disorders. Am J Ophthalmol 2004 Jan: 137(1):24-29.
14. Ossoining KC, Cennarmo G. Byrne SF. Echographic differential diagnosis of optic nerve lesions, in Thijssen JM, Verbeek AM (eds): Ultrasonography in Ophthalmology. Dordrecht, Dr. W. Junk, 1981:327.
15. Kurz-Levin MM, Landau K. A comparison of imaging techniques for diagnosing drusen of the optic nerve head. Arch Ophthalmol 1999:117:1045-9.
16. Gabbert-McConkiie W, Kachur KH. Diagnostic ultrasound in CPT expert. Salt Lake City: Ingenix 2006.
17. Weinstein SL. The cost of defensive medicine. AAOS Now 2008 Nov. Available at http://www.aaos.org/news/nov08/managing7.asp. Accessed December 14, 2009.
18. Lee AG, Brazis PW. The evaluation of eye pain with a normal ocular exam. Semin Ophthalmol 2003 Dec; 18(4): 190-9.
19. Brazie PW, Lee AG, Stewart M, Capobianco D. Clinical review: The differential diagnosis of pain in the quiet eye. Neurologist 2002 Mar;8(2):82-100.
20. Ossoinig KC. Standardized echography: Basic principles, clinical applications, and results. Int Ophthalmol Clin 1979;19:127.