Since its inception in the 1990s, optical coherence tomography has become a crucial tool in the practice of ophthalmology by informing diagnosis, disease monitoring and long-term prognosis. The ability of this technology to capture the peripheral retina has allowed for new and expanded clinical applications. In the course of its use and development, OCT technology has spawned widefield and ultra-widefield imaging methods that allow fields of view of up to 220 degrees. With the advent of these UWF imaging modalities, many researchers have initiated studies investigating the utility of UWF-OCT imaging. In this literature review, we’ll outline four disease entities in which UWF-OCT has shown promise: retinal detachments; pathological myopia; peripheral retinal degenerations; and choroidal pathologies, as well as highlight the uses of this modality in pediatrics and UWF-OCT angiography.
WF and UWF Defined
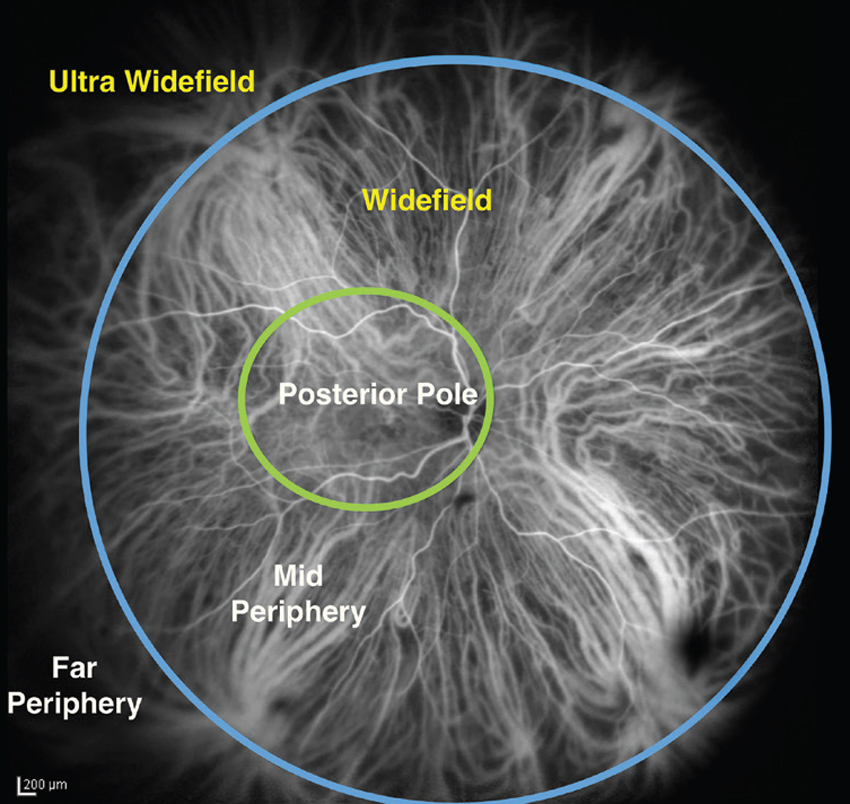 |
| Figure 1. A single image from the International Widefield Imaging Study Group demonstrating the definition of widefield and ultra-widefield with reference to the vortex vein ampullae. Demonstrated here are the demarcation boundaries described for the posterior pole, the mid-periphery and the far periphery. |
In 2019, the International Widefield Imaging Study Group defined widefield imaging as a field of view of approximately 60 to 100 degrees, capturing the mid-periphery of the retina up to the posterior edge of the vortex vein ampulla.1 It defined ultra-widefield imaging as an image of the far periphery of the retina, including the anterior edge of the vortex vein ampulla and beyond.1 This represents a 110 to 220-degree field of view. A depiction of these definitions appears in Figure 1.
Until recently, capturing the far periphery of the retina with OCT was nearly impossible. However, the Heidelberg Spectralis HRA-OCT (Heidelberg Engineering USA) (using a steering technique), the Silverstone (Optos PLC Edinburgh), the Plex Elite 9000 (Zeiss, Oberkochen, Germany) and the Xephilio OCT-S1 (Canon Medical Systems, Japan) have introduced UWF capabilities. A company called Toward Pi has also developed a swept-source OCT machine with an 81 x 68 degree field of view and an A-scan speed of 400 kHz.2
As mentioned, in the following sections we’ll look at the utility of WF and UWF in various conditions.
Retinal Detachment
There’s potential for the use of UWF-OCT in the diagnosis, monitoring, and management of retinal detachments. Microstructural retinal details such as photoreceptor integrity and resolution of subretinal fluid are difficult to ascertain on clinical exam or UWF fundus photography. UWF-OCT, however, acquires crucial information both before and after retinal detachment treatment. Toronto’s Wei Wei Lee, MD, and colleagues presented longitudinal findings captured by the Optos Silverstone that provided insight into the response of the retina to treatments including laser retinopexy and cryopexy.3 OCT findings post-cryopexy revealed separation of the choroid and sclera in the first week, a previously undescribed finding.3 Other OCT findings confirmed what’s been described in past histological analyses, including coagulative necrosis and retinal splitting after laser retinopexy, as well as retinal layer destruction and RPE separation post-cryopexy. A post-hoc analysis of the PIVOT trial comparing vitrectomy and pneumatic retinopexy for the treatment of retinal detachment examined postoperative outer retinal folds on OCT.4 They found that these ORF were associated with poorer visual outcomes at one year, and that those treated with vitrectomy were at greater risk of postoperative ORF.4 Although they evaluated OCT of only the posterior pole, these findings suggest that UWF-OCT in these patients would provide additional information about retinal healing after retinal detachment repair in the mid-far periphery where retinal breaks typically occur.
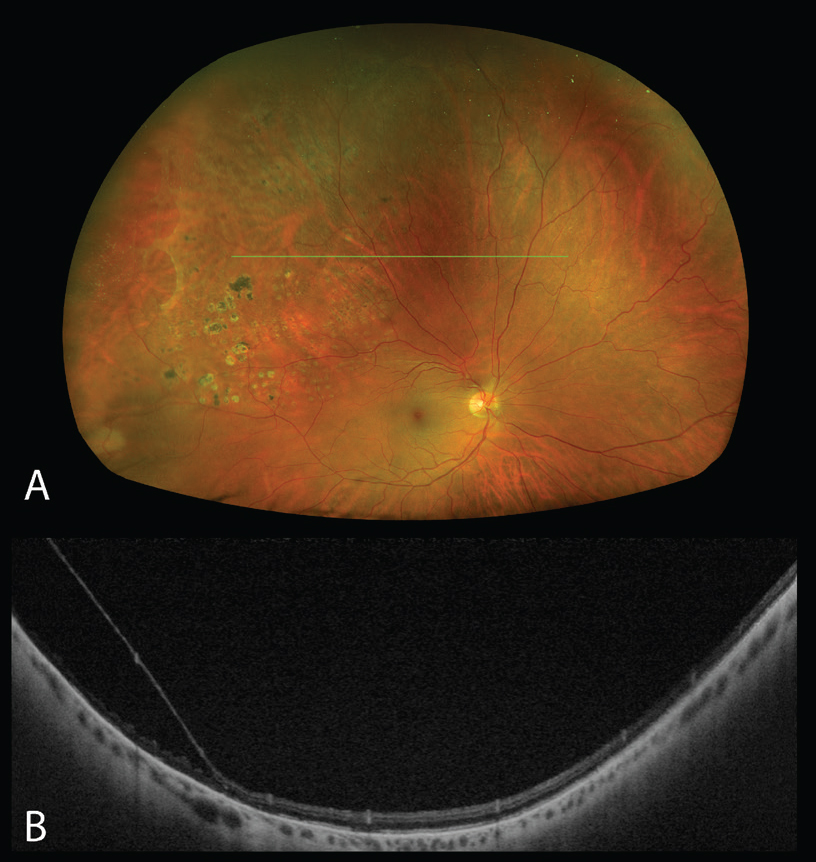 |
| Figure 2. Degenerative retinoschisis in the right eye captured using the Silverstone OCT (Optos, Edinburgh). (A) Pseudocolor image demonstrating a superotemporal area of retinoschisis, with translucency of the inner retinal layers and a reticular pattern of schisis cavities. Laser scars around the area of schisis are visible. (B) Swept source-OCT structural B-scan of the peripheral retina over the area of retinoschisis, revealing separation of the inner and outer retina consistent with retinoschisis. |
UWF-OCT can also aid in the differentiation of retinal detachments from degenerative retinoschisis or schisis detachments in cases where the clinical findings may be ambiguous (Figure 2).5–7 Cases clinically diagnosed as retinoschisis have been shown to have retinal detachment on OCT and vice versa.8,9 In 2014, Marilette Stehouwer and her colleagues at the Academic Medical Centre at the University of Amsterdam found that out of 18 presumed retinoschisis cases, three were shown to have retinal detachment on peripheral OCT, while another study reported a rate of six out of 53 eyes.6,9 This distinction is particularly relevant as the management for these two conditions differs significantly, with retinoschisis often being a benign condition requiring no intervention. However, one indication for intervention in retinoschisis is retinal holes, a finding which can also be captured on peripheral OCT.10 These preliminary studies show that UWF-OCT may be able to yield more useful information in the diagnosis and management of retinal detachment.
Pathologic Myopia
Since its development, UWF-OCT has been used to investigate and characterize features of high myopia, including posterior staphylomas, dome-shaped macula (DSM), and choroidal thickness, providing insight into classification and pathophysiology of these findings. Although DSM was originally considered a type of staphyloma, UWF-OCT has demonstrated that it’s distinct in its pathophysiology.11 DSM has been defined as an inward bulging of at least 50 µm involving the retinal pigment epithelium and Bruch’s membrane (Figure 3). Findings on UWF-OCT now suggest that DSM is related to an abnormal posterior scleral curvature.11
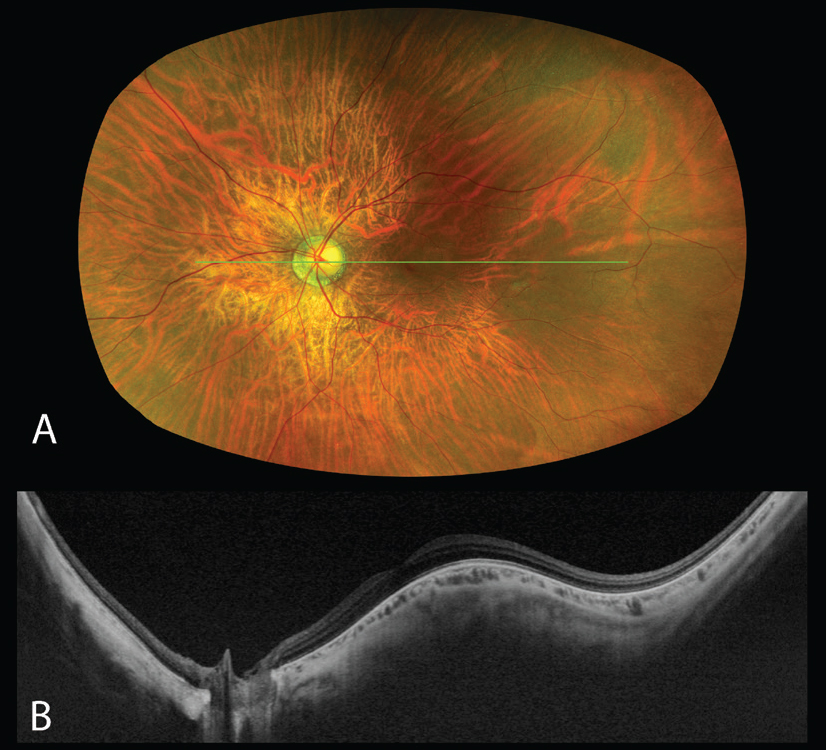 |
| Figure 3. Dome-shaped macula in the left eye captured using the Silverstone OCT (Optos, Edinburgh). (A) Pseudocolor image. (B) SS-OCT 24-mm structural B-scan passing through the optic nerve and fovea demonstrating the inward bulge of the dome-shaped macula. |
With regards to classification of staphylomas, UWF-OCT can aid in distinguishing wide and narrow varieties and allows for quantitative measurement of these staphylomas.12,13 In eyes with narrow staphylomas, Tokyo’s Noriko Nakao, MD, and her colleagues found that higher axial lengths were correlated with more abrupt staphyloma edges. This wasn’t the case with wide staphylomas, further differentiating these staphyloma categories.12 In another study, Tokyo’s Kosei Shinohara, MD, and co-workers reported that UWF-OCT may be more sensitive than 3D MRI in the detection of staphylomas, although the results weren’t statistically significant.14 Detection and monitoring of these staphylomas provide information about progression and risk of complications.
Peripheral Retinal Degeneration
Peripheral retinal degenerations are pathologies that may demonstrate the highest utility for UWF-OCT to date. UWF-OCT can provide clear characterization and documentation of these peripheral pathologies including lattice degeneration, retinal tufts, retinal tears, retinal holes and paving-stone degeneration. The feasibility of acquiring clinically useful OCT of these pathologies in practice has been demonstrated in several studies.5,15–17 In 2016, one of this article’s authors, Dr. Choudhry, used a steering technique to acquire UWF spectral-domain OCT of the peripheral retina and described structural features of these peripheral pathologies.17 In 2021, Simrat K. Sodhi and her co-authors at Vitreous Retina Macula Specialists of Toronto demonstrated that high quality and clinically valuable SS-OCT of the mid and far periphery could be captured without montage or steering.5 That same year, New York’s Kyle Kovacs, MD, and colleagues reported that the use of UWF-OCT provided meaningful clinical information to inform management in 38 percent of eyes imaged.15 This year, Paulo Eduardo Stanga, MD, and co-workers (one of whom is an employee of OCT-maker Canon Medical Systems) used a novel UWF-OCT device in a retrospective study to image pathology of the peripheral retina and were able to correlate findings with histological photomicrographs showing the retina and vitreous attachments.16 The researchers found that, in addition to microstructural details in the peripheral retina, OCT can also provide important information about the vitreoretinal interface and the presence or absence of traction. Such distinctions in pathology and associated features can help avoid invasive management by ruling out tears and holes in cases of vitreoretinal tufts or by ruling out vitreoretinal traction in cases of lattice degeneration.16
Choroidal Pathology
Choroidal pathologies are entities that can present in the peripheral retina where clinical exam alone may not be sufficient to make a diagnosis. UWF-OCT has been used to differentiate and diagnose choroidal melanoma and choroidal nevi in the retinal periphery.5 Important risk factors for transformation of nevi into melanoma include presence of subretinal fluid on OCT.18 In the case of peripheral lesions, UWF-OCT allows the detection of subretinal fluid and estimation of lesion size. Peripheral exudative hemorrhagic chorioretinopathy (PEHCR) lesions have been found to simulate the appearance of choroidal melanoma and are thus important to properly characterize. Since PEHCR lesions are usually located in the retinal periphery (89 percent between the equator and ora serrata), use of UWF or peripheral OCT is particularly valuable.19 The presence of retinal exudation and RPE atrophy can assist in differentiating PEHCR from choroidal melanoma.19 In a retrospective study of PEHCR lesions in 50 eyes of 35 patients, detection of subretinal fluid on OCT was a risk factor for future macular involvement, intravitreal bleed and loss of vision.19 Lesion extension beyond three clock hours also denoted high-risk eyes.19 Subgroup analysis from this study suggested that treatment of these high-risk eyes may protect against macular involvement.19 Continued research using peripheral OCT could further inform treatment recommendations.
Shanghai’s Yi Xuan, MD, and colleagues examined a series of choroidal osteomas using Toward Pi’s novel SS-OCT and OCTA technology, allowing an ultra-high resolution 120-degree field of view, capturing the entire tumor.2 This imaging modality was capable of detecting choroidal neovascularization, which can be difficult on traditional imaging modalities due to the dense nature of the mass and RPE changes.2
UWF-OCT in Pediatrics
Ophthalmic imaging in pediatric patients presents a unique challenge with regard to positioning and fixation. As in adults, subtle anatomic changes detectable with OCT imaging are clinically valuable in many conditions. One solution to address challenges with positioning is the use of handheld OCT devices. Thanh-Tin P Nguyen, MD, of Oregon’s Casey Eye Institute, and colleagues have shown the utility of a handheld SS-OCT device in non-sedated pediatric patients in the neonatal ICU, and in sedated patients in the operating room.20,21 Their widefield prototype device has a 105-degree field of view with the option of displaying real-time en-face OCT images.20,21
Particularly for pediatric conditions such as retinopathy of prematurity, this technology can play a role in screening and monitoring, and could even provide new pathophysiologic insights.20 With widefield OCT, the physician can determine the area of vascularized retina and the vascular/avascular border.20 The user can accurately detect and characterize neovascularization, particularly extraretinal neovascularization, which is important to the classification of ROP.20 Other clinically valuable OCT findings include changes in the vitreoretinal interface, which might inform management decisions in several pathologies. In pediatric retinal detachments, OCT findings can distinguish between tractional and exudative detachments.21 Objective OCT measures can also help monitor subtle changes over time.
Furthermore, widefield OCT can be valuable in cases of retinoblastoma, as tumors and subclinical-sized tumors can be detected in the retinal periphery.21,22 The utility of OCT in the detection of subclinical retinoblastoma tumors less than 400 µm, undetectable by ophthalmoscopy, has been well-established.22–24 Marie-Claire Gaillard, MD, and colleagues from the University of Lausanne in Switzerland, presented a case series of 16 subclinical recurrent tumors detected with a commercial handheld OCT device.23 Although this device didn’t have widefield capabilities, it demonstrated the benefit of OCT in the monitoring of patients with retinoblastoma. This may help detect recurrences earlier, which could have a significant impact on survival and visual outcomes. However, it can also provide important clinical details about the tumor, which may inform management decisions. The further evolution of pediatric widefield OCTA could prove valuable in the detection and management of intraocular tumors.
UWF-OCT Angiography
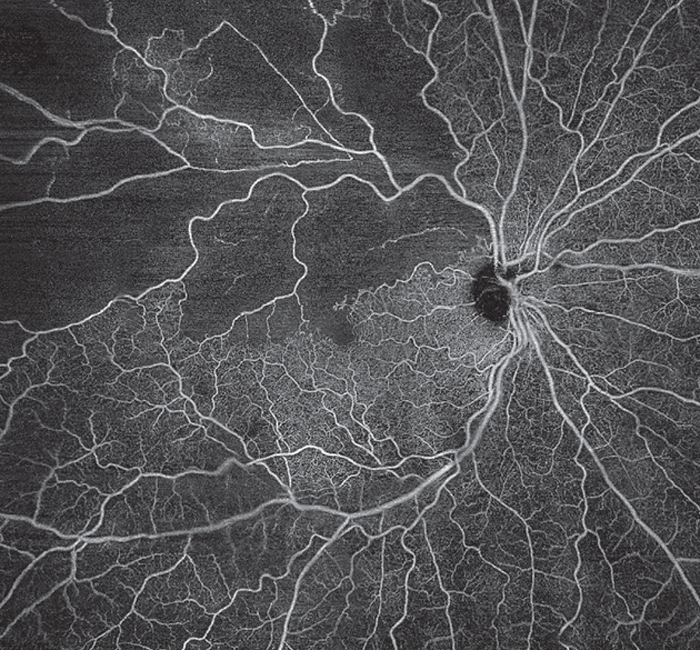 |
| Figure 4. Branch retinal vein occlusion in the right eye captured using the Plex Elite 9000 (Zeiss, Oberkochen, Germany). A 24 mm x 24 mm en face SS-OCTA montage of the superficial vascular plexus demonstrates areas of non-perfusion and disruption of the foveal avascular zone. |
OCT’s evolution has led to the development of non-contrast angiography capabilities. Though it’s currently not widely used, OCTA can be valuable as a non-invasive, safe and easily repeatable alternative to dye-based angiography with fluorescein or indocyanine green.25
An advantage of OCTA is the ability to create high-resolution, depth-resolved angiographic images, which can be correlated with flow overlay B scans.25 A major limitation of early OCTA technology was the limited field of view, but newer technology has allowed for wider field OCTA.26 Similar to OCT imaging standards, the International Widefield Imaging Study Group in 2019 recommended OCTA definitions for widefield and ultra-widefield. Widefield OCTA must capture all four quadrants of the retina including the posterior edge of the vortex veins, while ultra-widefield OCTA requires imaging beyond the anterior edge of the vortex veins. If not all four quadrants are captured, it must be labeled as asymmetric widefield, or asymmetric ultra-widefield, OCTA.1
One area in which widefield OCTA can be useful is in the evaluation of diabetic retinopathy. OCTA of the peripheral retina in DR can outline areas of non-perfusion and document vascular changes such as vessel pruning or neovascularization. The additional information afforded by OCTA may improve classification of diabetes severity. For example, in a retrospective, cross-sectional study, Fupeng Wang, PhD, of the University of Washington-Seattle, and co-workers used widefield OCTA to look at the ratio of nonperfusion (RNP) in eyes with diabetes without retinopathy, eyes with non-proliferative DR and eyes with proliferative DR.27 The RNP was significantly different between these groups. Interestingly, subgroup analysis suggested that nonperfusion in the peripheral retina (between 50 to 100-degree field of view) was the most valuable in grading DR severity.27
Differentiation of intraretinal microvascular abnormalities from neovascularization is also key in classifying DR severity. IRMA can be distinguished on widefield OCTA by the presence of intraretinal collateral vessels, with no flow signals above the internal limiting membrane.28 Presence of IRMA denotes severe NPDR and represents a high risk of progression to PDR.28 Although fluorescein angiography is considered the gold standard for detection of retinal neovascularization, two studies, one a cross-sectional study in 82 eyes by Francesco Pichi, MD, of the Cleveland Clinic Abu Dhabi and co-authors and a retrospective study in 82 eyes by Moorfields’ Hagar Khalid, MD, and co-workers, showed that the detection rate of NV on widefield OCTA may be better than detection on FA, color photography and clinical examination.26,29
Research has found clinical value in widefield OCTA assessment of retinal vein occlusions. In two studies (consisting of 43 patients and 26 patients, respectively), detection and quantification of areas of retinal nonperfusion with OCTA appears to correlate closely with FA.30,31 Since the peripheral retina often has larger areas of nonperfusion, widefield OCTA may allow a more accurate estimation of the extent of nonperfusion.31 In the first study, Agnès Glacet-Bernard, MD, and her colleagues at Paris-Est Créteil University in France, found that using a 60-degree field of view rather than a 12x12 40-degree field of view revealed nonperfusion in 30 percent more eyes.30 In a different study, Moorfields’ Josef Huemer, MD, and his co-authors used OCTA to describe different patterns of neovascularization in RVO: a sea-fan type and a nodular type.32 They noted a tendency for nodular neovascularization to be misdiagnosed as retinal hemorrhage on clinical exam, highlighting the clinical contribution of OCTA in these patients.32
In a retrospective study of 54 patients, Shanghai’s Wenyi Tang, MD, and colleagues found that the depth-resolved nature of OCTA allowed for evaluation of the periarterial capillary-free zone (paCFZ), which is the avascular area surrounding retinal arteries, measured in the superficial capillary plexus.33 This has been investigated as a potential biomarker in RVO, and has been shown to be larger in eyes with branch RVO.33 The researchers looked at this measure before and after anti-VEGF therapy and found an improvement in paCFZ with treatment.33 Dr. Tang’s group also found that lower ratio of paCFZ to artery area tend to predict better visual outcomes at 12 months with anti-VEGF injections.33
In conclusion, though it’s not currently widely used, UWF-OCT can be valuable in detection of pathology, such as subclinical retinoblastoma and diagnosis of clinically uncertain presentations, such as retinoschisis. It can play a role in monitoring of disease progression, such as myopic staphylomas, and inform treatment decisions through clear information about structural anatomy such as the vitreoretinal interface.
Despite significant improvements in technology, there exists many limitations to its application in practice. One obvious limitation is the cost of machines with widefield or ultra-widefield capabilities. Given the financial outlay required, consideration must be given as to the clinical utility of OCT and OCTA, as well as the other capabilities of these machines. For example, some devices offer ultra-widefield OCT capability, but can also be used to capture ultra-widefield pseudocolor photography, autofluorescence imaging, and fluorescein and indocyanine green angiography. Each clinician must evaluate the utility of these modalities in their practice. Another factor to consider is the challenges in image acquisition. Many artifacts must be managed with wider field imaging, such as eyelid artifact, inversion artifact and motion artifacts.
The majority of the scans also depend on the patient’s ability to fixate for longer periods of time, particularly with OCTA, as more information is being acquired. As machines evolve, the speed of acquisition increases, making this technology more useful in the ophthalmic population. Many commercial devices have an acquisition speed of 100 kHz (or greater), including the Optos Silverstone, the Plex Elite 9000, and the Xephilio OCT-S1 but the newer Toward Pi device and the prototype pediatric hand-held device both have an acquisition speed of 400 kHz.2,5,16,20,30
Looking ahead, as the existing platforms that can image the periphery become more readily available, these imaging modalities have the potential to play an important role in the growing field of telemedicine. Furthermore, the application of artificial intelligence on OCT and OCTA image processing, quantification and interpretation is a rapidly evolving field that could improve clinical management and prognostication for patients with central and peripheral retinal disease in a new era of personalized medicine.
Corresponding author:
Netan Choudhry MD FRCS(C)
Medical Director
Vitreous Retina Macula Specialists of Toronto
3280 Bloor Street West, Suite 310
Toronto, Ontario, M8X 3X3
Email: netan.choudhry@vrmto.com
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
Dr. Orr is a research fellow at Vitreous Retina Macula Specialists of Toronto and senior scientist at Toronto’s OCTane Imaging Lab.
Dr. Pereira is PGY3 at the University of Toronto Department of Ophthalmology & Visual Sciences and a senior scientist at the OCTane Imaging Lab.
Dr. Choudhry is co-founder and medical director at Vitreous Retina Macula Specialists of Toronto and principal investigator at the OCTane Imaging Lab.
Dr. Choudhry is a consultant for Topcon, Optos, Bayer, Allergan, Hoffman La Roche, Viatris, Novartis, Carl Zeiss Meditec, and Ellex and receives research equipment from Topcon, Optos & Carl Zeiss Meditec. This project was completed at the Vitreous Retina Macula Specialists of Toronto. The other authors have no financial interest in any of the material presented.
1. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: Recommendations from the International Widefield Imaging Study Group. Ophthalmol Retin 2019;3:10:843-849.
2. Xuan Y, Chang Q, Zhang Y, et al. Clinical observation of choroidal osteoma using swept-source optical coherence tomography and optical coherence tomography angiography. Appl Sci 2022;12:9.
3. Lee WW, Muni RH. Single-capture ultra-widefield guided swept-source optical coherence tomography in the management of rhegmatogenous retinal detachment and associated peripheral vitreoretinal pathology. Br J Ophthalmol 2022;0:1-7.
4. Lee WW, Bansal A, Sadda SR, et al. Outer retinal folds after pars plana vitrectomy vs. pneumatic retinopexy for retinal detachment repair: Post hoc analysis from PIVOT. Ophthalmol Retin 2022;6:3:234-242.
5. Sodhi SK, Golding J, Trimboli C, Choudhry N. Feasibility of peripheral OCT imaging using a novel integrated SLO ultra-widefield imaging swept-source OCT device. Int Ophthalmol 2021;41:8:2805-2815.
6. Eibenberger K, Sacu S, Rezar-Dreindl S, et al. Monitoring retinoschisis and non-acute retinal detachment by optical coherence tomography: Morphologic aspects and clinical impact. Acta Ophthalmol 2017;95:710-716.
7. McNabb RP, Grewal DS, Mehta R, et al. Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol 2016;100:10:1377.
8. Yeoh J, Rahman W, Chen FK, Da Cruz L. Use of spectral-domain optical coherence tomography to differentiate acquired retinoschisis from retinal detachment in difficult cases. Retina 2012;32:8:1574-1580.
9. Stehouwer M, Tan SH, Van Leeuwen TG, Verbraak FD. Senile retinoschisis versus retinal detachment, the additional value of peripheral retinal OCT scans (SL SCAN-1, Topcon). Acta Ophthalmol 2014;92:3:221-227.
10. Rachitskaya AV, Yuan A, Singh RP, Sears JE, Schachat AP. Optical coherence tomography of outer retinal holes in senile retinoschisis and schisis-detachment. Br J Ophthalmol 2017;101:4:445-448.
11. Saito R, Shinohara K, Tanaka N, Takahashi H, Yoshida T, Ohno-Matsui K. Association between dome-shaped macula and posterior staphyloma in highly myopic eyes investigated by ultra-widefield optical coherence tomography. Retin J Retin Vitr Dis 2021;41:3:646-652.
12. Nakao N, Igarashi-Yokoi T, Takahashi H, Xie S, Shinohara K, Ohno-Matsui K. Quantitative evaluations of posterior staphylomas in highly myopic eyes by ultra-widefield optical coherence tomography. Invest Ophthalmol Vis Sci. 2022;63
:8:1-8. doi:10.1167/IOVS.63.8.20
13. Ludwig CA, Moon J, Garg I, Miller JB. Ultra-widefield imaging for evaluation of the myopic eye. Semin Ophthalmol 2021;36:4:185-190.
14. Shinohara K, Shimada N, Moriyama M, et al. Posterior staphylomas in pathologic myopia imaged by widefield optical coherence tomography. Investig Ophthalmol Vis Sci 2017;58:9:3750-3758.
15. Kovacs KD, Mahrous MA, Gonzalez L, et al. Feasibility and clinical utility of ultra-widefield–navigated swept-source optical coherence tomography imaging. J Vitreoretin Dis 2021;5:5:396-404.
16. Stanga PE, Pastor-Idoate S, Reinstein U, et al. Navigated single-capture 3D and cross-sectional wide-field OCT of the mid and peripheral retina and vitreoretinal interface. Eur J Ophthalmol. 2022;32:3:1642-1651.
17. Choudhry N, Golding J, Manry MW, et al. Ultra-widefield steering-based sd-oct imaging of the retinal periphery HHS public access. Ophthalmology 2016;123:6:1368-1374.
18. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019;39:10:1840-1851.
19. Zicarelli F, Preziosa C, Staurenghi G, Pellegrini M. Peripheral exudative haemorrhagic chorioretinopathy: A widefield imaging study. Br J Ophthalmol 2021;105:1410-1414.
20. Nguyen TTP, Ni S, Khan S, et al. Advantages of widefield optical coherence tomography in the diagnosis of retinopathy of prematurity. Front Pediatr 2022;9:1-7.
21. Nguyen TP, Ni S, Liang G, et al. Widefield optical coherence tomography in pediatric retina : A case series of intraoperative applications using a prototype handheld device. Front Med 2022;9:1-12.
22. Skalet AH, Campbell JP, Jian Y. Ultrawide-field OCT for retinoblastoma. Ophthalmology 2022;129:6:718.
23. Gaillard MC, Houghton S, Stathopoulos C, Munier FL. OCT-guided management of subclinical recurrent retinoblastoma. Ophthalmic Genet 2018;39:3:338-343.
24. Berry JL, Cobrinik D, Kim JW. Detection and intraretinal localization of an “invisible” retinoblastoma using optical coherence tomography. Ocul Oncol Pathol 2016;2:148-152.
25. Spaide RF, Fujimoto JG, Waheed NK, et al. Optical coherence tomography angiography. Prog Retin Eye Res 2018;64:1.
26. Pichi F, Smith SD, Abboud EB, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefe’s Arch Clin Exp Ophthalmol 2020;258:1901-1909.
27. Wang FP, Saraf SS, Zhang Q, et al. Ultra-widefield protocol enhances automated classification of diabetic retinopathy severity with OCTA. Ophthalmol Retin 2020;4:4:415-424.
28. Arya M, Sorour O, Chaudhri J, et al. Distinguishing intraretinal microvascular abnormalities from retinal neovascularization using optical coherence tomography angiography. Retin J Retin Vitr Dis 2020;40:9:1686-1695.
29. Khalid H, Schwartz R, Nicholson L, et al. Widefield optical coherence tomography angiography for early detection and objective evaluation of proliferative diabetic retinopathy. Br J Ophthalmol 2021;105:1:118-123.
30. Glacet-Bernard A, Miere A, Houmane B, et al. Nonperfusion assessment in retinal vein occlusion: Comparison between ultra-widefield fluorescein angiography and widefield optical coherence tomography angiography. Retina 2021;41:1202-1209.
31. Kadomoto S, Muraoka Y, Uji A, et al. Nonperfusion area quantification in branch retinal vein occlusion: A widefield optical coherence tomography angiography study. Retina 2021;41:1210-1218.
32. Huemer J, Khalid H, Wagner SK, et al. Phenotyping of retinal neovascularization in ischemic retinal vein occlusion using wide field OCT angiography. Eye 2021;35:2812-2819.
33. Tang W, Liu W, Guo J, et al. Wide-field swept-source OCT angiography of the periarterial capillary-free zone before and after anti-VEGF therapy for branch retinal vein occlusion. Eye Vis 2022;9:25:1-10.



