Though intraocular lenses have been around a long time, the market continues to grow and improve each year as new innovations are made. Patients who undergo cataract surgery are offered a host of IOLs for implantation, such as monofocal, multifocal and extended depth-of-focus lenses. But what if there was an IOL that could precisely mimic the human crystalline lens? Here, we take a look at several of the accommodative IOL concepts in development.
Accommodation: The Elusive Target
There have been many theories in the past that explained how the eye can be restored after surgery with accommodative effort. To create a truly accommodative IOL, researchers and pharmaceutical companies have been developing lenses using hypotheses from these accommodative theories. One of the first and best-known theories to propose accommodation in the eye was that of Hermann von Helmholtz.
In von Helmholtz’s hypothesis, he suggests that during distance vision, the ciliary muscle is relaxed and the zonules are in a state of “resting” tension. When an accommodative IOL (A-IOL) is introduced into the eye, the ciliary muscle can contract and release the tension of the zonules. When this occurs, the accommodative power of the lens increases.1 This theory provides a basic blueprint on how A-IOLs can be developed and used in surgery, but the road to true accommodation isn’t easy.
“The promise of accommodation continues to be attractive although it has been challenging to mimic true human accommodation, which is far more complex than [von] Helmholtz originally predicted,” says George Waring IV, MD, the founder and medical director of the Waring Vision Institute in South Carolina. “However, this hasn’t held back innovation, and in recent years there have been numerous accommodative IOLs in development.”
When will an A-IOL meet true accommodation and gain market approval from the FDA? “Proving accommodation is tricky because there’s no gold standard as to what the FDA wants with respect to an endpoint for accommodation, and how do you actually prove accommodation as the mechanism?” asks Sumit Garg, MD, a cataract surgeon at UCI Health in Irvine, California. “The FDA is in the process of updating their requirements on what’s required to designate an IOL as ‘accommodating,’ ” Lenses currently undergoing development are being held back by this, but it doesn’t mean the future of A-IOLs isn’t bright.
“I think the future is bright for IOL technology in general, particularly accommodative technology,” comments Dr. Waring. “I don’t believe we’ll be able to reproduce the young human crystalline lens, but we’ll get to the next best thing. But, like all technologies, this takes time to develop and will continue to evolve and improve overtime.”
• Juvene (LensGen). One novel device on its way to FDA trials is LensGen’s Juvene A-IOL. “They have a modular IOL that has shown the ability to achieve accommodation,” says Dr. Garg. “The question is: Is it going to meet what the FDA requires to be called accommodation versus some other designation which would have an equivalent refractive effect?
“LensGen has shown best corrected distance corresponding intermediate and near vision without diffractive optics,” continues Dr. Garg. “But, to actually prove that’s happening is more difficult because there’s no real guideline on how to show that and, typically, the movements required to give this range of vision are really small and therefore hard to capture.”
  |
|
A simulated image of the Juvene lens implanted into the eye. The dioptric power of Juvene is adjusted through the curvature change influenced by the ciliary muscle. This A-IOL is implanted into the capsular bag. (Courtesy LensGen) |
Dr. Garg presented the 36-month visual outcomes after implantation of the Juvene A-IOL at the 2023 ASCRS meeting in San Diego. The data presented focused on 10 eyes with seven various diopter points examined between the 24-month follow-up and the 36-month follow-up visits. Researchers observed the means of best-corrected distance visual acuity (-0.06 logMAR [slightly better than 20/20]), distance corrected intermediate (0.09 logMAR [slightly worse than 20/20]) and near visual acuities (0.21 logMAR [around 20/32]), as well as binocular measures for intermediate (-0.02 logMAR [around 20/20] ) and near (0.12 logMAR [a little worse than 20/25]).2
Also presented at the meeting were monocular defocus curves that showed that visual acuity was better than 20/40 from +1.5 D through -2.5 D of defocus. Binocular defocus curves increased the diopter range from +2 D through -2.5 D. Additionally, contrast sensitivity curves were reported to be “virtually identical” to a monofocal lens.
“We haven’t seen any safety signals that said that this isn’t going to be a very safe lens inside the eye,” says Dr. Garg. At the ASCRS presentation, he reported that the Juvene A-IOL didn’t show any device-related adverse events during follow-up visits after a three-year period. He adds, “We have toxicology studies to satisfy FDA requirements, so there’s no issues with biocompatibility with the material.”
The Juvene lens is developed using a two-part system. “It has a fixed optic in the base that fills the whole capsular bag and then it has a fluid lens optic that fits within that base to give you that actual change in refraction needed to give you continuous vision from distance, intermediate and near,” explains Dr. Garg. “So, you put the base lens in first and then you put in the fluid lens, and then you have to tab it into place inside the eye. It’s not as simple as putting it in the eye and then you’re done. There’s a little bit more to it than a standard lens. That being said, I’ve done a handful of them outside the U.S. and it’s not a very difficult thing to do.
“Because it’s a two-part lens, there’s some modularity to it,” continues Dr. Garg. “So, you can always exchange or upgrade the optic, if you needed to, depending on if a patient’s vision requirements change.”
Dr. Garg mentioned that the Juvene lens is implanted using an off-the-shelf injector but didn’t disclose precisely what injector system he’s used in the past. However, he did add that the incision for implanting the A-IOL is much larger than a typical incision needed for other lenses. “It’s a little over a 3-mm incision for the injection into the capsular bag,” he says.
Currently, LensGen is preparing their lens for their Phase I FDA trial. “The hope is that everything will go well there to move towards commercialization in a few years assuming no major hiccups,” comments Dr. Garg.
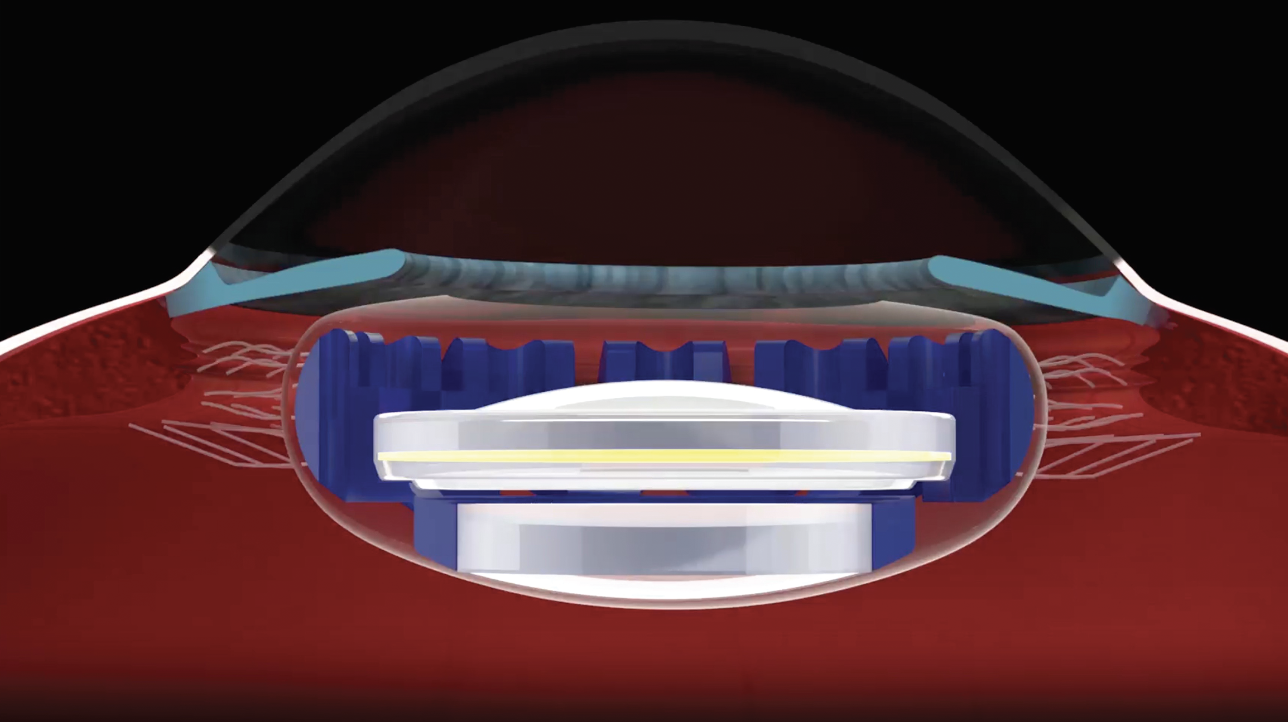
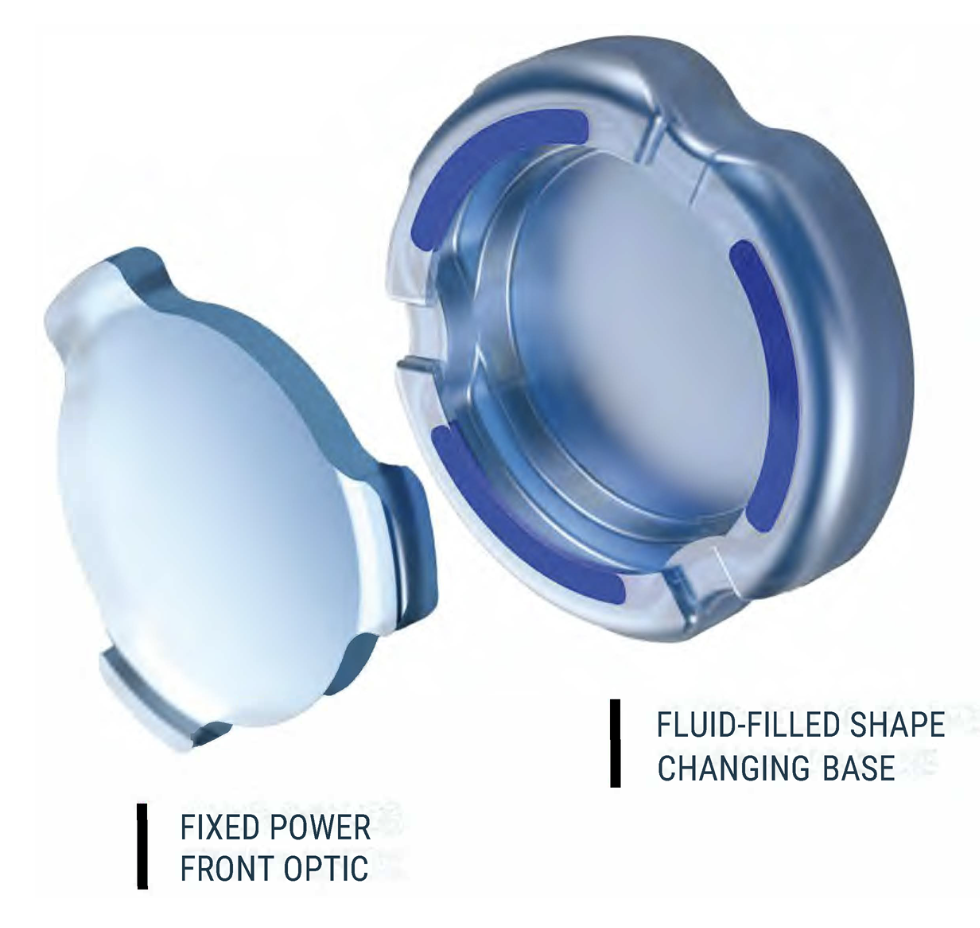
• OmniVu (Atia Vision). “The OmniVu is a modular shape-changing lens system that’s composed of a fixed power front optic and a fluid-filled shape changing base,” explains Dr. Waring. “The fixed power front optic is a hydrophobic acrylic and the shape changing base is fluid-filled. This is designed to restore a continuous full range of vision binocularly across more than four diopters of defocus while promoting contrast sensitivity and without unwanted dysphotopsias or other photopic side effects.
“Six months and one year in-human trials have been presented,” adds Dr. Waring. “The six-month data showed that 95 percent of eyes were within a half a diopter of plano and maintained greater than 20/32 visual acuity over four diopters of defocus binocularly. It will come in a power range of +12 to +28 in half-diopter increments and recently, 12-month data has also been presented at AAO by Daniel Chang, [MD].”
 |
|
OmniVu’s fluid-filled shape changing base is inserted first into the capsular bag followed by the fixed power front optic, which docks into the base. (Courtesy Atia Vision) |
Dr. Chang’s presentation at AAO 2023 provided one-year follow-up results from 13 eyes (monocular) and four patients (binocular) with the OmniVu implanted. He reported that after one year, 92 percent of eyes were within a 0.5 D of plano. Additionally, he reported that monocular CDVA, DCIVA and DCNVA were −0.06 ± 0.08 [better than 20/20], 0.01 ± 0.10 [around 20/20] and 0.19 ± 0.12 [around 20/32]. Furthermore, binocular CDVA, DCIVA and DCNVA were −0.15 ± 0.10 [better than 20/16], −0.02 ± 0.07 [better than 20/20] and 0.15 ± 0.12 [a little worse than 20/25]. In the presentation, Dr. Chang reported that the mean monocular defocus curves showed visual acuity better than 20/32 from +1.25 D through -1.75 D, and binocular defocus curves showed the same visual acuity from +1.75 D through -2.75 D of defocus. Further data was reported at the AAO meeting.3
Dr. Waring goes on to explain the OmniVu’s surgical technique. “This is done with a standard phaco technique,” he says. “The lens does require a 3.5-mm incision and a 5.5-mm capsulotomy. The base lens is inserted into the capsular bag after standard phacoemulsification and the fixed power front optic is then injected also into the capsular bag and is docked with the docking tabs into the base.
“This lens is designed to provide a continuous range of vision and [Atia Vision] believes that the unique features of the capsule-filling technology may provide additional beneficial characteristics such as stabilization of effective lens position with the bag-filling technology, as well as potentially minimizing posterior capsule opacification,” continues Dr. Waring. “Even though the lens appears to provide a full range of vision, there’s always the possibility of requiring magnification, such as a small amount of magnification for reading details and low-light conditions.”
There are other developments for the OmniVu planned as well. “The toric option is planned for the future and another unique attribute of this technology is the fact that the front optic can be changed if needed for refractive purposes or other enhancements in the future,” says Dr. Waring.
• FluidVision (Alcon/PowerVision). In 2018, Louis Nichamin, MD, an ophthalmologist from Brookville, Pennsylvania, presented the results from a six-month pilot study on the FluidVision A-IOL.4 During the presentation, Dr. Nichamin explained that the FluidVision A-IOL is developed with a refractive-index-matched, silicone fluid-filled optic which is connected by channels to two fluid-filled haptics. It achieves accommodation by forcing fluid from the haptics into the optic, which increases the thickness of the lens as well as the optical power. To reverse the accommodation, fluid flows back into the haptics.
Dr. Nichamin mentioned that the pilot study focused on monocular results in 28 subjects. Only one subject wasn’t included for the six-month follow-up visit. Each subject had a FluidVision A-IOL implanted through a 3.5-mm incision and their visual acuities along with contrast sensitivity were assessed. The mean CDVA at six months was -0.05 logMAR [a bit better than 20/20], the mean DCIVA was 0.05 logMAR [a little worse than 20/20], and the mean DCNVA was 0.14 logMAR [a little worse than 20/25], respectively. Also, he mentioned that the subjects achieved contrast sensitivity equivalent to a monofocal lens.
In 2019, Alcon announced that it would be acquiring PowerVision to develop the FluidVision A-IOL. According to PowerVision, future planned developments include introducing a toric platform and improving post-implant adjustment. They also believe the incision can be smaller, and PowerVision is looking into reducing the size to 2.8 mm.5
• Opira (ForSight Vision6). According to the company, the Opira A-IOL is a silicone, ciliary muscle driven, capsule-fixated, dynamic shape-changing device. It’s attached to the capsule using the A-IOLs haptics.6
David F. Chang, MD, a cataract surgeon in Los Altos, California, provided the latest trial results for the Opira A-IOL at Hawaiian Eye 2024. During the six-month study, a total of 32 subjects had the Opira implanted bilaterally through a 3.75-mm incision. Only two subjects weren’t included in the results. In logMAR notation, monocular visual acuity at distance, intermediate and near were -0.02 (a little better than 20/20), -0.07 and 0.04 (a little worse than 20/20). Binocular visual acuity at distance, intermediate and near were -0.06, -0.11 (a little better than 20/16) and -0.01 logMAR. Uncorrected visual acuity at distance, intermediate and near were -0.04, -0.11 and -0.02 logMAR with 97 percent of subjects becoming spectacle-free.
Then, subjects were asked to note any adverse outcomes in a questionnaire following the study. Dr. Chang reported that adverse effects such as glare, starbursts, hazy vision, distortion, focusing difficulties, depth perception issues, as well as fluctuations in vision, were all lower in comparison to monofocal and trifocal counterparts. It was reported that a total of 275 eyes have been implanted with the Opira A-IOL over the last five years, with no cases of uveitis-glaucoma-hyphema syndrome, IOL dislocation or IOL explantation.
Dr. Chang reported in his presentation that while the A-IOL is fixated to the capsule, the mechanism for accommodation is independent of the capsular fibrosis which allows for more predictable performance. A future plan for Opira is to reduce the incision size for implantation to 3 mm.
 |
| According to AkkoLens, the Lumina can provide patients with continuous “sharp” vision from 25 to 40 cm. (Courtesy AkkoLens) |
• Lumina (AkkoLens). According to AkkoLens, the Lumina is a two-piece A-IOL consisting of an anterior element and a lens. This lens is implanted within the sulcus to promote accommodation. Research has suggested that the design of the Lumina A-IOL can provide approximately 4 D of accommodative power. It requires a 2.8 mm incision, which makes the procedure suture-free.6
In a study to analyze the accommodative performance of Lumina, researchers compared the lens to a monofocal IOL.7 A total of 25 eyes were implanted with Lumina and 18 eyes received a monofocal lens as a control. After a one-year follow-up, researchers observed that Lumina subjects had better visual acuity results than subjects in the control group over a defocus range of -0.5 to -5 D. The study showed that Lumina had similar UDVA results to the control group. However, Lumina showed significantly better UNVA of 0.91 ± 0.11 (20/22 Snellen) compared to the control group.
• JelliSee (JelliSee Ophthalmics). JelliSee is currently undergoing human pilot studies. According to their website, the A-IOL’s design is foldable and offers 7 D or more of accommodation. When the ciliary muscle in the eye relaxes, a force is applied by the zonules to the lens. Only <0.2 mm of diameter change in the eye is needed to gain full range of accommodation. The haptics of the A-IOL are fixated to the fibrotic peripheral lens capsule to allow the natural accommodation mechanism to work effectively, the company says.
During the 2021 Winning Pitch Challenge presented at ASCRS, representatives for JelliSee explained that the lens is liquid-filled with a relatively flat anterior surface. Due to the haptics, a radial outward force is applied to the anterior surface.
 |
|
JelliSee Ophthalmic’s A-IOL is a two-piece, silicone-based IOL implanted into the capsular bag. (Courtesy JelliSee Ophthalmics) |
Other A-IOLs
Not all A-IOLs are undergoing human trials. There are some lenses in development that are currently going through biocompatibility studies. Liliana Werner, MD, PhD, a researcher and director of the Intermountain Ocular Research Center at the University of Utah, provided Review with information about the latest devices undergoing biocompatibility studies using rabbit models.
“The company Adaptilens is developing what they call a biomimetic accommodating lens,” informs Dr. Werner. “The design was conceived to imitate the elastic, young natural crystalline lens, and it’s composed of a membrane filled with a proprietary high molecular weight polymer engineered with specific chemical, optical and mechanical properties to mimic human crystalline lens properties. The lens has been tested in cadaver eyes with capsular bags of varying sizes, demonstrating at least 4 D of accommodation. It uses the natural mechanism of accommodation of the eye. Current pre-clinical studies performed in the rabbit model in our laboratory are demonstrating biocompatibility and safety.
“The company Ocumetics is developing the Bionic lens,” continues Dr. Werner. “This is a one-piece, silicone, air-filled shape-changing lens. There’s an air-filled space between the optical elements; there’s no fluid such as silicone oil within the lens. It can be inserted through a 3-mm incision, and it’s fixated within the capsular bag; it was designed to deliver 3.25 D of accommodation. The processes of accommodation and dis-accommodation happen fast, in less than a second. Biocompatibility is being evaluated in our lab in rabbit studies. During accommodation, with ciliary body contraction and zonular relaxation, there’s an increase in the anterior-posterior dimension of the lens, increasing IOL power. And, during dis-accommodation, with ciliary body relaxation and increase in zonular tension, the anterior-posterior dimension decreases, decreasing the IOL power.”
Future of A-IOLs
Once accommodation of a sort can be achieved in these lenses, the market is going to begin opening up. Unfortunately, there are several lenses in development and only a few researchers have had the opportunity to study and work with them. Should young ophthalmologist be worried about learning the particular implantation techniques for these lenses? Probably not, since both Dr. Waring and Dr. Garg agree that there’ll only be a small learning curve when implanting these lenses.
“I think time will tell what will happen as we get further into trials to figure out what actual learnings there are for the particular lenses, and I’m sure there’ll be nuances for each lens depending on if it’s one-piece or two-piece, where you have to place it and how it gets assembled,” explains Dr. Garg. “So, certainly it will be something that’ll require some training, like any new technology does. We learned that with MIGS and we learned that with other intraocular surgeries such as secondary lens implantation. There’s aways new skillsets that we’re learning. I don’t anticipate this being any different than that.
“Do I think that it’ll be a very high bar to achieve? I don’t,” continues Dr. Garg. “I think most of these lenses are going to be fairly straightforward, but I don’t think it’s going to be the same as just implanting a one-piece into the capsular bag.”
Dr. Waring adds, “I think the learning curve will be modest. It’s part of the innovation cycle where we optimize techniques. The first-generation technology inevitably has enhancements over time for ease of use and increased efficacy. So, yes, we do anticipate a modest adoption period and optimization process over time which is in line with new technology employment.”
Eventually, these devices will undergo FDA trials with market approval in their sights. It seems that ophthalmologists have been anticipating this technology since it was first theorized. “As they are essentially monofocal lenses, accommodating IOLs are still considered the holy grail of presbyopia correction,” says Dr. Werner.
Surgeons are hopeful for the future of lens replacement surgery as more accommodating IOLs begin development. “Our hope is that different lenses make it to market that can build one on top of the other to give us options for our patients,” says Dr. Garg. “Our goal as a surgeon is to have choices for our patients so that we can tailor the best lens to meet their needs.”
Dr. Waring is an investigator for Atia Vision. Dr. Garg is an investigator for LensGen. Dr. Werner is an investigator for Alcon/PowerVision, LensGen, Atia Vision, Adaptilens and Ocumetics.
1. Chapter 9: Accommodative and nonaccommodative treatment of presbyopia. 2020-2021 BCSC Basic and Clinical Science Course. 2021;13:9. https://www.aao.org/education/bcscsnippetdetail.aspx?id=43fd133d-1389-44d7-8be2-e2db1dda79cf#:~:text=The%20Helmholtz%20hypothesis%20or%20capsular,state%20of%20%E2%80%9Cresting%E2%80%9D%20tension.
2. Garg S. Thirty-six-month visual outcomes after implantation of modular, shape-changing, fluid-optic intraocular lens. ASCRS ASOA Annual Meeting. May 6, 2023. https://ascrs.confex.com/ascrs/23am/meetingapp.cgi/Paper/90906.
3. Chang DH. First-in-human clinical feasibility study of a dual-optic accommodating IOL system. AAO Annual Meeting. November 5, 2023. https://secure.aao.org/aao/meeting-archive?_gl=1*o7i5nk*_ga*NjkzNzU5MjMuMTcwODQ1MjU4NA..*_ga_3PN52QWGQQ*MTcxMDM0NTU4MC40LjEuMTcxMDM0NTY2Ni40NC4wLjA.
4. Nichamin L. New IOLs: Material & preload IOL. 36th Congress of the ESCRS. September 24, 2018. https://legacy.escrs.org/vienna2018/programme/free-papers-details.asp?id=30251&day=0.
5. Cheskin B. FluidVision: Designed to restore true accommodation. Ophthalmology Innovation Source. October 13, 2016. https://ois.net/fluidvision-designed-to-restore-true-accommodation/.
6. Naseri A. Accommodating IOL. ASCRS Annual Meeting. July 26, 2021. https://ascrs.confex.com/ascrs/21am/meetingapp.cgi/Paper/79746.
7. Rombach M. The AkkoLens Lumina accommodative lens. Acta Ophthalmologica 2014;92:0-0.
8. Alió JL, Simonov AN, Romero D, et al. Analysis of accommodative performance of a new accommodative intraocular lens. J Refract Surg 2018;34:2:78-83.