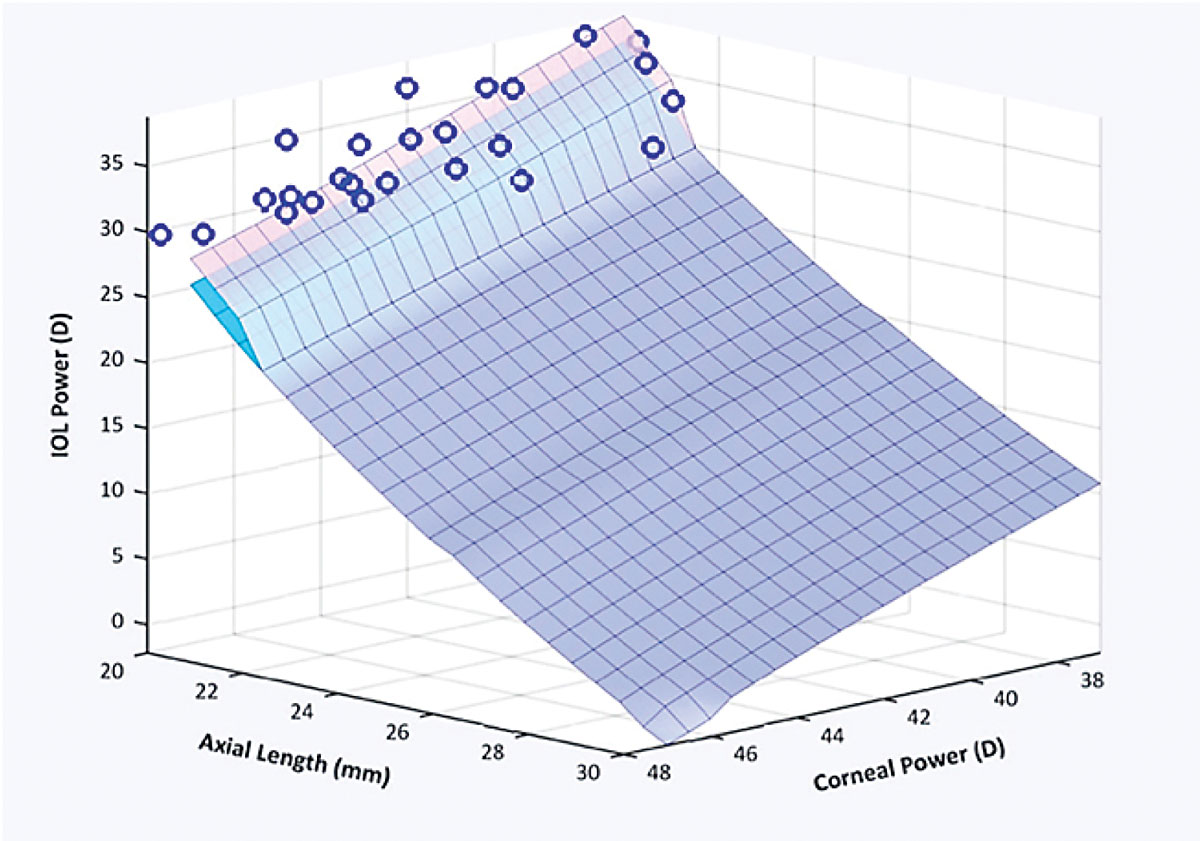
Refractive cataract surgery is an ever-evolving field. Biometric measurements are getting more accurate; our understanding of which anatomic factors affect lens position continues to improve; patient expectations continue to increase; and formulas for calculating lens power keep getting more sophisticated—and are starting to rely on artificial intelligence.
Here, experts share the latest information and advice.
Which Optical Biometer?
With many optical biometers available, does it matter which one you use? “Today we have a dozen different types of biometry units that can provide reliable information, including the recently introduced Heidelberg Anterion and the Alcon Argos,” notes Terrence P. O’Brien, MD, the Charlotte Breyer Rogers Distinguished Chair in Ophthalmology and director of the Refractive Surgery Service at Bascom Palmer Eye Institute of the Palm Beaches. “That’s good, because competition has forced manufacturers to keep improving their instruments.
“Here at Bascom Palmer we’re fortunate to have multiple instruments available,” he says. “In our experience, these modern biometers are all very accurate in terms of measuring axial length, with a very small standard deviation. However, there’s still room for improvement when measuring corneal power—we do see some disagreement there.”
Uday Devgan, MD, FACS, FRCS, chief of ophthalmology at Olive View UCLA Medical Center, a clinical professor at the UCLA School of Medicine, and in private practice at Devgan Eye Surgery in Los Angeles, says he doesn’t believe that one optical biometer will produce significantly better outcomes than another. “Doing the best possible lens power calculation is more important,” he says. “The difference in axial length measurements between machines is on the order of one or two hundredths of a millimeter—about 10 or 20 µm. Even if two biometers come up with slightly different axial length measurements, the difference will be negligible. The lens power’s going to end up the same.
“Some instruments make additional measurements, such as Zeiss’s True Cornea measurement,” he continues. “That could make a difference for some patients, but I’m not convinced it will make a significant difference overall. Comparing the optical biometers is like comparing a Mercedes, a BMW and an Audi. You may prefer one over another, but they’re pretty similar. Getting the lens power calculations right will make a much bigger difference than refining those measurements or adding new ones.”
Making Sense of the Formulas
“It’s somewhat daunting to figure out which formula will give you the most reliable data for IOL power and toric placement,” notes Dr. O’Brien. “For practical reasons, I understand that many colleagues use one or two formulas exclusively; some will only check multiple formulas if the axial length is unusually long or short. In any case, the available formulas are improving over time. The Barrett Universal was a game changer for many people; that’s one that some surgeons have migrated to as their only formula. Warren Hill has the RBF, which is excellent, and he constantly refines it as he gets more data.
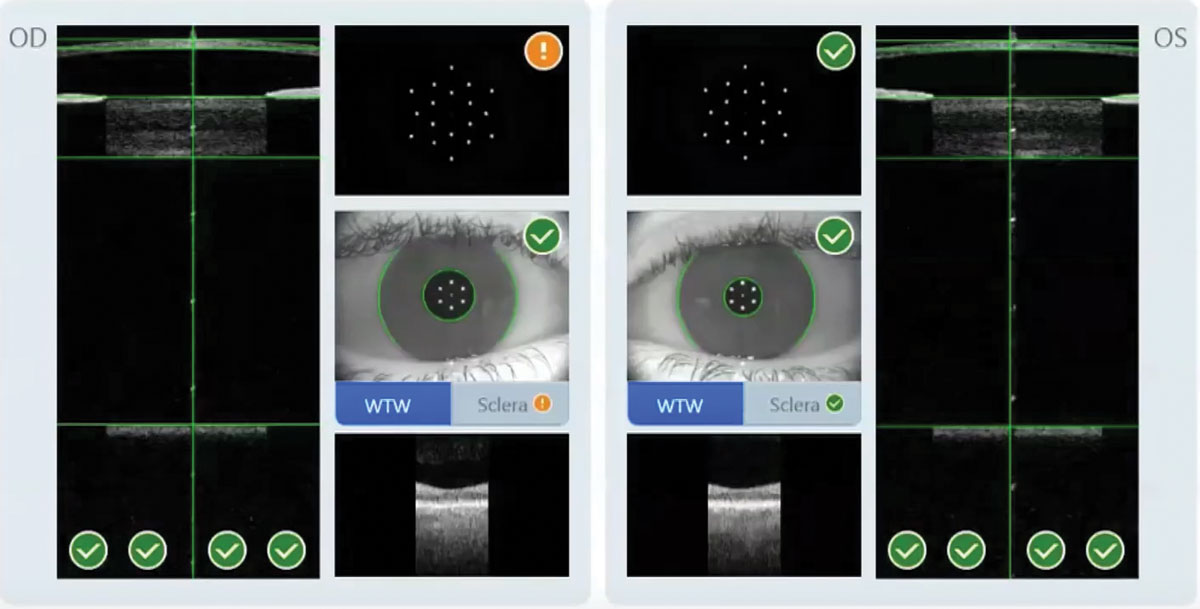 |
|
Figure 1. Zeiss says the IOLMaster 700 can measure both eyes in 45 seconds. Here, the system flags suspicious keratometry. |
“I still enter my data into several formulas and compare the results,” he continues. “In addition to the Barrett and Hill RFB, I learned a lot from Dr. Jack Holladay, and I still use his Holladay 1 and 2 formulas. For me, just seeing the variability between formula results allows me to start zeroing in on where I want to be. A lot of variability can alert me that there may be something abnormal, such as a shallow anterior chamber or a dense cataract. You can also use the results from multiple formulas to help educate the patient. The fact that the formulas are not totally in agreement helps to keep the patient’s expectations realistic.”
Dr. O’Brien says that if he gets widely variable answers from different formulas, he goes back and looks at the raw data to make sure there wasn’t a problem. “For example, we take multiple measurements using topography and other devices such as the Pentacam,” he says. “If a Pentacam taken on one day and an optical biometry scan taken on another day are quite different, that makes me wonder if the patient’s eyes were drier on one day than the other.
| Measuring the posterior corneal surface “Most surgeons understand that measuring the power of the back surface is potentially useful information,” says Jack Holladay, MD, MSEE, FACS, president of Holladay Consulting in Houston and the developer of the Holladay 1, 2 and Refractive Formulas. “On average, the back of the cornea reduces the total corneal power by about 12 percent. For example, for a 7.5-mm anterior radius cornea with a keratometric power of 45 D, the front surface power would be 50.13 D and the average back surface power would be 6.49 D, with a net power of 43.78 D. Every lens power formula uses an average index of refraction to compensate for this reduction in total power of the cornea. But if the back surface isn’t average, that can result in an error when calculating the lens power that’s needed.” How often will it make a significant difference in our lens power calculation? “Studies show that adding the posterior measurement doesn’t make very much difference in 80 percent of the population, because their back corneal surface is pretty much average,” he notes. “But 20 percent have unusual back surfaces; in those patients you may get a better result because you took the back surface into account. And of course, you can’t know which one in five patients will benefit from the additional measurement, so ideally you should always measure it.” Dr. Holladay adds an important consideration. “If you do measure the actual power of the posterior corneal surface, you don’t want to start including that adjustment before plugging your numbers into the formula,” he says. “The formulas already make an adjustment for that. To avoid this problem, the latest technologies that measure the back surface are simply adjusting the reported power to account for how far above or below normal the back surface is. For example, Zeiss has now incorporated OCT into the IOLMaster 700 so that it can measure the posterior cornea. What they call “Total K” is the keratometric K adjusted by how far the back surface measurement is from average (plus or minus). “The other manufacturers are working on adding this capability to their instruments, too,” he notes. “Meanwhile, you can now measure the astigmatism on the posterior corneal surface as well as the spherical component, which can lead to a more accurate measurement of net astigmatism. Measuring the back of the cornea will help improve outcomes, but it’s still early in the game.” —CK |
“If you don’t want to use more than one or two formulas,” he adds, “I’d suggest plugging your data into the Barrett Universal 2 and the Hill RBF. If those two are in reasonable agreement, you can be pretty confident your refractive outcome will be close to what you anticipated.”
To understand why some formulas are more successful with certain eyes, it helps to know a little about the evolution of these formulas. Jack Holladay, MD, MSEE, FACS, president of Holladay Consulting in Houston and the developer of the Holladay 1, 2 and Refractive Formulas, explains that a number of factors led to many early formulas having trouble avoiding refractive surprises in long or short eyes. “When optical biometry began to appear around 2000, Wolfgang Haigis, PhD, was very instrumental in the calibration of the IOLMaster,” Dr. Holladay says. “He measured thousands of eyes to accomplish this, but none of them were longer than 26 mm, and only a few were shorter than about 20 mm. So he extrapolated his measurement curves in both directions.
“The problem with extrapolation,” Dr. Holladay continues, “is that it’s not based on data. All of the early formulas were developed using ultrasound-measured Ks. When the IOLMaster came out in 2000, clinicians began to switch to that, but nobody realized that Wolfgang had extrapolated those long- and short-eye values. Then about 10 years ago, Doug Koch, MD, and Li Wang, MD, showed that the measurement conversion between ultrasound and optical biometry isn’t a straight line. For example, someone with a long eye might get a 36-mm measurement using the IOLMaster, while ultrasound would measure that eye as 34 mm—the correct measurement. The error gets progressively worse the longer the eye. Meanwhile, the other optical biometers were calibrated to the IOLMaster, so they all had the same problem.
“Drs. Koch and Wang then published a regression that can be used to correct for this error,” he adds. “Today, the more recent formulas, such as the Barrett, Olsen and Holladay 2, compensate for this, so clinicians don’t need to use a conversion factor to compensate for it themselves.”1
Office Setup for Success
Surgeons offers these suggestions to help ensure better outcomes:
• Take the time to learn your biometry machine. Dr. O’Brien points out that many clinicians don’t really know much about the details of using the optical biometers. “Most of us haven’t even learned the basics of what they can do—and more importantly, what it looks like when they don’t do things properly,” he says. “That’s why we have our fellows work with experienced technicians and do biometry on a few patients from scratch. We want them to appreciate all of the steps along the way, and the variables that can lead to less-precise data. Most of our fellows tell us later that it was a really valuable experience.”
• Train several technicians to be experts. “Many practices will designate one person to do this, and cross-train someone else as a backup person,” notes Dr. O’Brien. “The thing is, you need to have a technician who is so familiar with the test that he or she can perform it in their sleep. Then, when something in the data is off, they sense right away that it may be unreliable.
“I suggest training several people to become real experts in using whichever biometer your practice has,” he concludes. “That’s the only way to ensure that all readings are of acceptable quality.”
• Use the latest technology. “Our IOLMaster 700 provides an OCT of the macula along with the data,” Dr. O’Brien points out. “That allows us to confirm that the patient was properly fixating, and we can see the fovea and make sure the contour is correct. We also may detect pathology such as an epiretinal membrane, vitreomacular traction or cystoid macular edema.”
• Be alert for potentially problematic patients. “Extreme refractive errors are a red flag,” Dr. O’Brien notes. “Someone who’s a -18-D myope is likely to have very abnormally long axial length and possibly a staphyloma. In those cases, you’re going to want to get not only A-scan but B-scan ultrasound readings to make sure you’re measuring properly, and not measuring in the canyon of a staphyloma in the posterior segment. In eyes that are challenging—the high myopes, extreme hyperopes, and patients who’ve had prior LASIK or RK—more information is better.”
Getting Accurate Measurements
These tips can help:
• Always measure both eyes. “This is important because the second eye serves as a check for the first eye,” notes Dr. Devgan. “The eyes should produce similar physical measurements; if they don’t, you need to stop and look carefully at how the data was collected and the condition of the eyes.”
• If you need to use ultrasound, measure both eyes and compare the result to your optical biometry measurement in the better eye. “Some patients have such a dense cataract in one eye that you can’t get an optical biometry measurement because light can’t get through,” notes Dr. Devgan. “In that situation, you have to do an A-scan ultrasound. Here again, you want to take this measurement in both eyes. If the measurements are close, that’s a good sign that the measurements are accurate. But even more important, if the other eye can be measured with optical biometry, you can compare the A-scan measurement to the optical measurement. If they agree, that tells you that you’re A-scan technique was good.”
• Make sure the ocular surface is pristine. “Getting an accurate corneal power is especially important because it changes the lens power in almost a one-to-one ratio,” Dr. Devgan points out. “If your corneal measurement is off by 1 D, your lens power will be off by 1 D—and the measured corneal power can be drastically different if the ocular surface isn’t in good shape.
“A problem could be as simple as untreated dry eye,” he continues. “It could be epithelial basement membrane dystrophy; it could be a pterygium on the cornea; it could be someone who wears contact lenses full time and just removed them five minutes before your exam, leaving the cornea warped. All of these can play a role in the accuracy of the K-reading, and that can change your lens calculation dramatically.”
“You probably don’t need to treat every patient’s ocular surface,” notes Dr. O’Brien. “However, you should check for surface problems in every patient—especially those who may be at higher risk because of age, gender, prior eyelid surgery or systemic medications known to adversely affect tear production or the ocular surface, as well as those patients who’ve had prior refractive surgery, which can affect innervation and corneal sensation. And you should have a low threshold for treatment; if you find something that’s less than ideal, take the time to address it before taking the biometry measurements. Treating the ocular surface for a few weeks is preferable to having a postoperative surprise.”
• Take your measurements on an eye that hasn’t yet been touched. “In some practices, a patient’s IOP is checked before the measurements are taken for the IOL power calculations,” notes Dr. Devgan. “That’s a mistake. Getting the IOP involves touching the eye and disturbing the epithelium, and that can alter the corneal measurement you get afterwards, throwing off your lens power calculation. In our clinic, everyone seeing me for a cataract consult has biometry measurements done before entering the exam room to have their vision and pressure checked.”
| Artificial intelligence joins the fray A notable trend in lens power formula development is the addition of artificial intelligence to the equation. Uday Devgan, MD, FACS, FRCS, chief of ophthalmology at Olive View UCLA Medical Center and a clinical professor at the UCLA School of Medicine in Los Angeles, is part of the group headed by John Ladas, MD, PhD, that’s been developing an artificial intelligence approach to lens power calculations known as the Ladas Super Formula 2.0. (Dr. Devgan also has an ownership interest in the company developing it.) “Today, many surgeons use Barrett and Hill RBF,” Dr. Devgan notes. “But for me, the most accurate formula is the Ladas Super Formula 2.0, which we’ve worked on for more than 20 years. The power is calculated using artificial intelligence, based on data from the more than 4,000 ophthalmologists who use IOLcalc.com. Most surgeons using this AI-based system are reporting 94- to 95-percent accuracy.
“This system should be available in clinical machines in the next few years,” he adds. “But right now, if you’re willing to enter your data manually, you can use this software to calculate your lens power at IOLcalc.com.” [A video explaining the Ladas Super Formula 2.0 can be viewed at cataractcoach.com/2021/06/26/1146-the-future-of-iol-calculations/.] —CK |
Verifying Data Quality
“When people refer patients to me because of a poor outcome, the biggest mistake often turns out to be the quality of the individual measurements on which the lens choice was based,” says Dr. O’Brien. “I think it’s extremely important for surgeons to verify that the quality of the data is good—especially the corneal power reading. There are a number of ways to do this:
• Look at the reflection of the LED mires on the cornea. “If they’re not sharp, you’re going to have garbage in, garbage out,” Dr. O’Brien says.
• Check the standard deviation.
“These devices also give us a standard deviation for each measurement,” notes Dr. O’Brien. “That helps us know if it falls within acceptable limits. For corneal power, we say a standard deviation greater than 0.3 D is a red flag; when determining the meridian for astigmatic correction, a standard deviation greater than 3.5 degrees is a red flag. In my experience the IOLMaster 700 and the Lenstar measure the axial length very precisely, and in most patients the corneal power is within those parameters as well. However, you have to check to be sure.”
• Get a second measurement. “This is another way to verify the accuracy of the measurement if there’s any doubt about it,” says Dr. O’Brien. “If possible, I’ll use a second instrument. However, even two measurements from the same device can be valuable, especially if you take them on two different occasions. Changing factors—such as the condition of the patient’s ocular surface—can affect the reading. Once you have a second reading, check to verify the consistency of the data. This is especially helpful if you’re implanting a toric lens.”
Choosing the Right Lens Power
Surgeons offer these tips to minimize postop refractive surprises:
• If choosing between two lens powers, err on the side of myopia. “If you’re faced with two slightly different power calculation outcomes—or you have one answer that falls between two lens powers—choose the higher lens power,” says Dr. Devgan. “That way, if you’re off, you’ll err on the side of slight myopia. A small amount of myopia postop isn’t a bad thing; it increases your range of vision in the intermediate zone.
“The other reason for doing this is the ease of resolving a problem if you end up with one,” he continues. “Let’s say you do end up with a myopic outcome; you aimed for plano and ended up at -1 D. If the patient doesn’t like being -1 D, that’s a slam dunk to fix, an easy LASIK or PRK treatment. But if you aim for plano and end up +1, hyperopic PRK or LASIK isn’t nearly as stable or accurate as myopic PRK or LASIK. Myopic PRK or LASIK takes away central corneal tissue, but hyperopic LASIK or PRK steepens the central cornea by lasering away a doughnut shaped section of tissue. That’s much less accurate.”
• When dealing with eyes that have had previous refractive surgery, consider using the ASCRS website calculator. “Today it’s quite common to do cataract surgery on eyes with prior LASIK, PRK or even RK,” notes Dr. Devgan. “There’s no one amazingly accurate way to measure these eyes. However, the American Society of Cataract and Refractive Surgery has an online calculator that lets you plug in whatever data you have, and it will use whichever of the 20 or so different formulas that are available to do the calculations. If you don’t have all of the data, it will use the formulas that are compatible with whatever data you do have. It will give you a spread of answers, rather than one conclusive answer. Again, I suggest erring on the side of ending up slightly myopic, by choosing a higher lens power.” [You can access the calculator at ascrs.org/tools/post-refractive-iol-calculator.]
• Be aware that there are two different types of short eyes. Dr. Holladay points out another factor that clinicians may not be aware of: There are two very different types of eyes that measure as having a short axial length. Identifying which you’re treating can significantly impact the accuracy of a refractive outcome.
“One type of short eye is a nanophthalmic eye, where the anterior segment is proportional to the smaller axial length,” he explains. “Then, there are axial hyperopes with a very short posterior compartment and axial length, but a perfectly normal anterior segment. Eyes with a short axial length are only nanophthalmic about 20 percent of the time; the other 80 percent are axial hyperopes. If you’re operating on an axial hyperope, you’ll need a lens with more power because the lens will sit deeper in the eye; if it’s a nanophthalmic eye, you’ll need less power, because the lens will sit closer to the cornea.
“In a paper my colleagues and I wrote back in 19962 we showed that the best measurement to use to make this distinction was the white-to-white measurement,” he continues. “Normal eyes, including axial hyperopes, had 12-to 12.5-mm corneal diameters, while nanopthalmic eyes had 9- to 10-mm diameters. So there’s a big difference between a nanophthalmic eye and an axial hyperope in terms of this measurement. Furthermore, the nanophthalmic eye often had a steep K and shallower anatomic anterior chamber depth, while the axial hyperope did not. So, those three dimensions of the eye are critical in differentiating whether you’re dealing with a nanophthalmic or an axial hyperope eye. That’s the reason the optical biometry companies began to add white-to-white, anterior chamber depth and lens thickness measurements, and why the newest formulas take that into account.”
Dr. Devgan has an ownership interest in Advanced Euclidean Solutions, the company behind IOLcalc.com and the Ladas Super Formula, and in CataractCoach.com, a free teaching website. Dr. Holladay is a consultant for Carl Zeiss, M&S Technologies, Oculus, Sonomed, Acutome, Visia Imaging, Zeimer Ophthalmics, Heidelberg Engineering, Medisoft Imaging and Ellex. Dr. O’Brien reports no financial conflicts.
1. Wang L, Holladay JT, Koch DD. Wang/Koch axial length adjustment for the Holladay 2 formula in long eyes. J Cataract Refract Surg 2018;44:10:1291-1292. Errata: January 2019 JCRS Jan 45 117.
2. Holladay JT, Gills JP, Leidlein J, Cherchio M. Achieving emmetropia in extremely short eyes with two piggyback posterior chamber intraocular lenses. Ophthalmology 1996;103:7:1118-23.