Even when a business is running smoothly, sometimes it can pay to re-engineer the way things are done—or look at the same old processes in a new, fresh way—in an effort to get even better results. Similarly, clinicians who treat glaucoma get very good results with their current medication options. But would it be useful to take a different approach and try to make use of different molecular pathways in the eye to give a positive jolt to outcomes? Currently, prostaglandin analogs are the most prescribed drug class for lowering intraocular pressure in glaucoma patients. Though PGAs work well, some patients may find they need combination therapies or multiple prescriptions to achieve desirable outcomes, which can make adherence even more of a challenge than usual.
Currently, only one of the glaucoma medications far along in the drug-development pipeline is a prostaglandin. The remaining therapeutics in the pipeline attempt to re-engineer clinicians’ approach to treatment, and feature novel mechanisms of action intended to tackle the disease differently than any of the interventions used today.
To learn about some of the innovative IOP-lowering drugs you may soon be able to offer your patients, read on.
NCX 470 (Nicox SA)
The PGA that has shown promise in clinical trials thus far is NCX 470, formed from a nitric oxide-donating compound. A previous animal study demonstrated the solution’s superiority over bimatoprost in lowering IOP and treating both glaucoma and ocular hypertension.1 More recently in 2021, the company reported the outcomes of the dose-response Phase II trial (Dolomites), which showed that patients on the novel drug experienced significantly greater IOP lowering effects than those on latanoprost.
In the Phase II trials, topical NCX 470 was administered using 0.065% concentration, while the commonly prescribed concentration of 0.005% was used for latanoprost. The company reported that NCX 470 was superior at all time points over the 28-day period and effectively lowered patients’ IOP levels by up to 1.4 mmHg more than latanoprost.
“NCX 470 is a nitric oxide-donating prostaglandin analog similar to Vzyulta,” says James C. Tsai, MD, MBA. “It could be slightly stronger than Vzyulta, which itself offers approximately 1 mmHg better pressure reduction than latanoprost, but I don’t believe that the 1.4 mmHg reduction with NCX 470 versus latanoprost will be a huge difference compared to Vyzulta’s effects.”
The once-daily dosed drop is currently being evaluated in two multi-regional Phase III clinical trials, known as Mont Blanc and Denali, which are expected to enroll 670 patients each. The primary objective is to demonstrate whether the efficacy of the 0.1% solution surpasses that of latanoprost 0.005% for reducing IOP in glaucoma patients. Investigators anticipate that the higher drop concentration than that previously used in trials may offer even greater IOP-lowering benefits without increased risk.
The company expects to publish the results from the Mont Blanc trial in the first quarter of 2023, and results from the Denali trial are expected by the end of that same year.
 |
| Researchers are experimenting with different pathways for treating glaucoma medically. |
Cromakalim prodrug 1 (CKLP1)
One of two prospective medications to use a new mechanism of action involving the reduction of episcleral venous pressure (EVP) is cromakalim prodrug 1 (CKLP1), an ATP-sensitive potassium channel opener being studied by a group of researchers from the United States and the United Kingdom. Glaucoma specialists like Dr. Tsai are particularly excited about the prospect of a drop that, for the first time ever, may offer patients clinical results equal to what used to be possible only through surgical intervention.
“If you lower the EVP by 2 mmHg, then the eye pressure is lowered by 2 mmHg,” explains Dr. Tsai. “If you’re working with a drug that reduces the aqueous flow or aqueous outflow, it’s not the same one-to-one relationship as EVP reduction. That’s why we in the glaucoma community are so excited about these new medications that target EVP.”
Dr. Tsai explains that with prostaglandins, there’s often a floor effect on IOP that prevents the pressure level from going below that of the EVP. “Right now with medication therapy, you can’t get a patient’s IOP to be under 8 to 10 mmHg,” he says. “But, with these drugs that are able to reduce EVP, we might be able to achieve pressures lower than that without having to perform surgery.” This eliminates the potential complications of surgery and replaces them with a medication that’s just taken once a day and can be discontinued if there’s an issue, Dr. Tsai adds.
Though this IOP-lowering drug has yet to be tested on humans, a study published last year observed its effect on large normotensive animals and found that CKLP1 was able to significantly lower IOP by 18.9 percent in monkeys and 16.7 percent in dogs compared with control eyes.2 The drug was also shown to have no effect on the animals’ systemic blood pressure.
Omidenepag isopropyl (Omdi, Santen)
This drug has been sold in Japan and other parts of Asia since 2018 but is still in the process of gaining FDA approval.
Omdi is a selective, non-prostaglandin, prostanoid EP2 receptor agonist. Its safety and efficacy have been shown in various studies, one which found that Omdi 0.002%, alone or administered concomitantly with timolol 0.5%, resulted in sustained IOP reduction through 52 weeks in Japanese patients with open-angle glaucoma or ocular hypertension.3
Another recent clinical trial including 190 patients concluded that Omdi 0.002% was well-tolerated and noninferior to latanoprost 0.005% in reducing IOP in patients with ocular hypertension or primary open-angle glaucoma.4 Though no serious side effects were reported, the most common side effect observed was conjunctival hyperemia, an interesting finding considering it’s often associated with prostaglandin agents. Although 24.5 percent of subjects reported hyperemia, not all the research agrees on the frequency of its occurrence.
The most recent study of the drug was published this past March and, according to the authors, found that “Omdi showed an IOP-lowering effect in eyes with various types of glaucoma and using various therapeutic regimens in real-world clinical practice.”5 Out of the 827 patients who participated in the study, 14 percent experienced some form of an adverse reaction, the most common being hyperemia (7.6 percent—much less common than observed in the previous study). There were also no serious side effects reported.5 Based on these positive outcomes, the study authors say that Omdi has the potential to be a first-line treatment for glaucoma.
“Because it’s a non-prostaglandin, it doesn’t have the same side effect profile as a prostaglandin would,” says David Solá-Del Valle, MD. “For instance, it doesn’t seem to have cystoid macular edema, periorbital atrophy or the periorbital pigmentation side effect profile. I have a growing group of patients who get all these side effects from latanoprost even though their IOP is good, so it would be nice to have the option to switch them to Omdi. I’m very excited about it.” He mentions the added benefit of once nightly administration, which may also improve adherence to treatment and help reduce damage to the ocular surface.
The FDA accepted the New Drug Application in February 2021 and is currently conducting the review process to investigate the drug as a treatment for patients with glaucoma and ocular hypertension.
QLS-101 (Qlaris Bio)
Further along in the pipeline than CKLP1, QLS-101 is the second ATP-sensitive potassium channel opener intended to target the reduction of EVP. The drug is currently in a Phase II clinical trial (Study QC-201) that’s testing three different concentrations vs. timolol maleate preservative free 0.5% in a cohort of 84 patients with POAG or ocular hypertension.
“QLS-101 is a novel ATP-sensitive potassium channel modulator administered as a topical eye drop,” says Dr. Tsai. “It works similar to CKLP1, in that it reduces EVP and widens outflow channels and episcleral vessels distal to the trabecular meshwork.”
In addition to potentially serving as an alternative noninvasive treatment to surgery, both QLS-101 and CKLP1 may also spare patients from the side effects seen with prostaglandins. Results from Study QC-201 will help define treatment outcomes and the risk of adverse events for one of the first drugs to use this new mechanism of action.
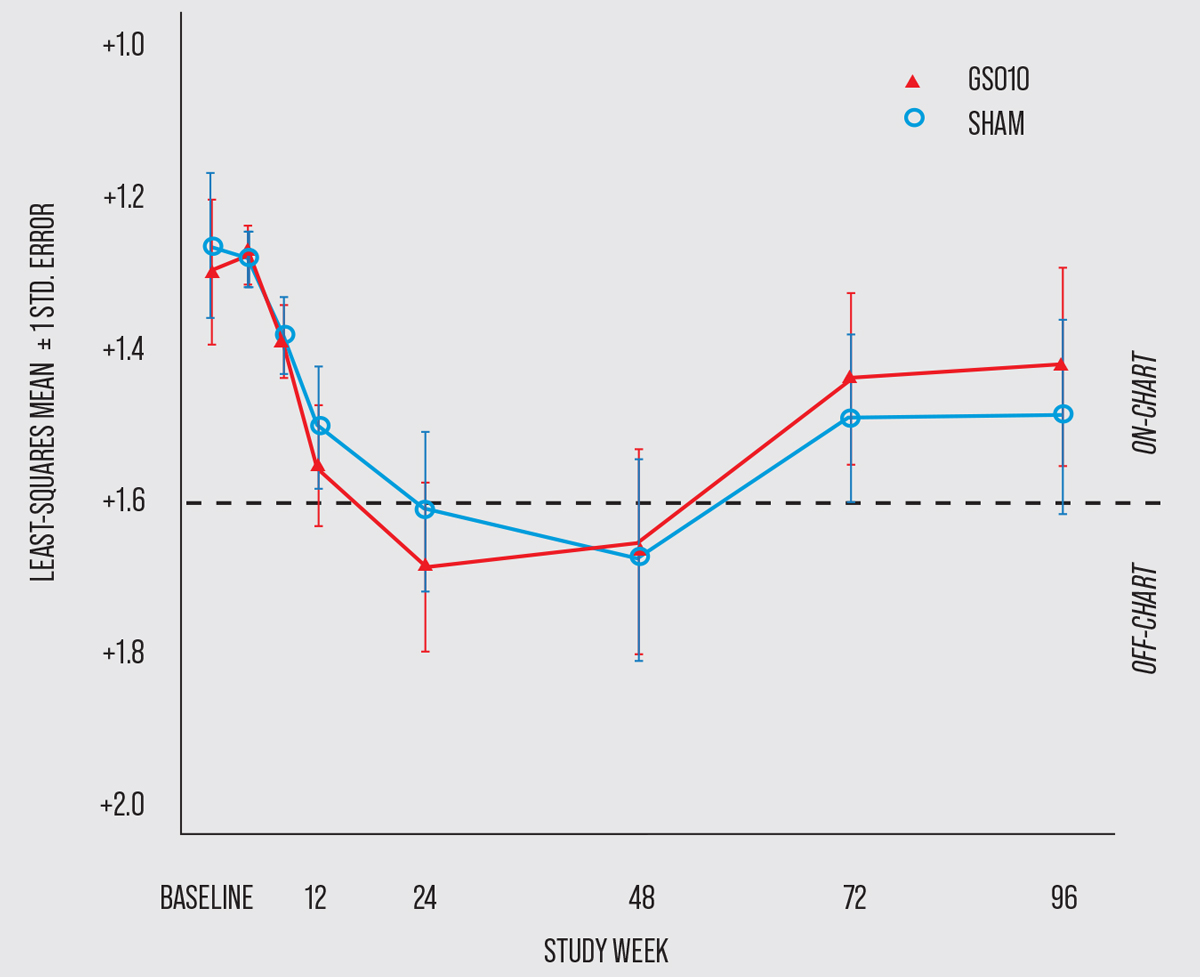 |
| This graph shows the time course of BCVA (in logMAR) of patients with LHON who participated in the 96-week RESCUE trial evaluating the developing gene therapy, GS010. (Click image to enlarge.) (Photo courtesy of GenSight Biologics.) |
GS010 (rAAV2/2-ND4, GenSight)
Though not specifically formulated or indicated for treating glaucoma, GenSight is investigating the potential role of GS010 in treating recent vision loss in patients with Leber hereditary optic neuropathy (LHON). The positive results so far show promise for the future of gene- and cell-based treatments for other neuro-ophthalmic conditions, including glaucoma.
The first Phase III trials of this novel gene therapy were recently completed in 2020 (RESCUE and REVERSE trials). The drug, administered via a one-time unilateral intravitreal injection, is specifically made for those with a G11778A mutation in the mitochondrial ND4 gene who sustained a recent loss of vision (within the previous six months for the patients in the clinical trial).
In the REVERSE trial, participants were randomly assigned to receive a sham injection in one eye and an injection of GS010 in the other. The results showed that visual improvement was sustained through the 96-week follow-up period in both eyes. At the conclusion of the trial, eyes treated with GS010 demonstrated a mean improvement in best-corrected visual acuity of -0.31 logMAR, while eyes treated with the sham injection showed a mean improvement of -0.26 logMAR. The insignificant difference in visual outcomes between the two groups meant that the primary endpoint wasn’t met, though 78 percent of subjects did show bilateral improvement in vision.6
The RESCUE trial produced comparable results; the difference of the change in BCVA from baseline between GS010-treated and sham-treated eyes was -0.01 logMAR, which fell short of the primary endpoint of a difference of at least -0.30 logMAR. The average BCVA of study participants decreased through week 24 and then peaked before plateauing up until week 48. By the end of the trial at week 96, eyes treated with sham injections shared a similar outcome to those treated with GS010.7
Though the therapeutic needs validation through additional research, these two Phase III trials have laid the groundwork for future studies to investigate the role of this modality in treating various forms of optic nerve disease.
In conclusion, there are several noninvasive therapeutic options you can look forward to possibly offering your patients in the future, including the topical formulations NCX 470, CKLP1, OMDI and QLS-101. In addition, other nonsurgical options such as gene therapy are showing positive preliminary results in clinical research. Not only do most of these glaucoma treatments have promising side-effect profiles, but some physicians say they may be able to provide patients with visual outcomes comparable to or better than those of minimally invasive glaucoma surgeries.
Dr. Solá-Del Valle is a member of the Glaucoma Service at Mass Eye and Ear in Boston, where he specializes in glaucoma care and the surgical treatment of glaucoma. He has consulted for Allergan. Dr. Tsai is the president of New York Eye and Ear Infirmary of Mount Sinai. There, he also serves as the Delafield-Rodgers Professor of Ophthalmology at the Icahn School of Medicine and System Chair of Ophthalmology for the Mount Sinai’s Health System. Dr. Tsai serves as a consultant for Eyenovia, ReNetX Bio and Smartlens.
This article has no commercial sponsorship.
1. Impagnatiello F, Bastia E, Almirante N, et al. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br J Pharmacol 2019;176:8:1079-1089.
2. Roy Chowdhury U, Kudgus RA, Holman BH, et al. Pharmacological profile and ocular hypotensive effects of cromakalim prodrug 1, a novel atp-sensitive potassium channel opener, in normotensive dogs and nonhuman primates. J Ocul Pharmacol Ther 2021;37:5:251-260.
3. Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: The RENGE study. Jpn J Ophthalmol 2021;65:6:810-819.
4. Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: The phase 3 AYAME Study [Published correction appears in Am J Ophthalmol 2021 Nov;231:211]. Am J Ophthalmol 2020;220:53-63.
5. Miki A, Miyamoto E, Ishida N, Shii D, Hori K; LESPOIR Research Group. Efficacy and safety of omidenepag isopropyl 0.002% ophthalmic solution: A retrospective analysis of real-world data in Japan Adv Ther. [Epub March 14, 2022].
6. Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med 2020;12:573:eaaz7423.
7. Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within six months of disease onset. Ophthalmology 2021;128:5:649-660.




