New Options Call for Better Communication
Diabetic eye disease is a leading cause of blindness among adults. Among diabetics over the age of 40 years, the estimated prevalence of diabetic retinopathy is approximately 40 percent, with 8 percent of subjects developing vision-threatening retinal complications.2
The standard treatment for diabetic macular edema (DME) is focal photocoagulation, the efficacy of which was determined in the Early Treatment Diabetic Retinopathy Study (ETDRS).3 However, attempts to treat refractory and persistent edema have led to new treatment options under evaluation. Intravitreous and peribulbar corticosteroid injections have been used in recent years to reduce macular edema, but large scale prospective studies have yet to be published regarding safety and efficacy. An exploratory, Phase II, randomized, controlled trial with the anti-VEGF aptamer Macugen suggested that inhibiting VEGF may be beneficial in the management of DME,4 but long-term studies of safety and efficacy of anti-VEGF agents in the treatment of DME are lacking.
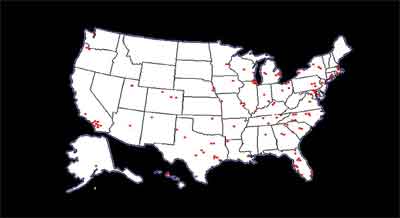
Figure 1. Participating sites of the Diabetic Retinopathy Clinical Research Network.
Some of the ongoing studies of the DRCR.net are described below. Each one aims to elucidate some aspect in the treatment of diabetic retinopathy, which is becoming more complex as treatment options increase.
Currently Recruiting Studies
• Evaluation of Vitrectomy for Diabetic Macular Edema Study. This is a prospective, non-randomized treatment study, the goal of which is to identify subgroups of subjects with DME for whom vitrectomy may or may not be beneficial. The outcomes to be evaluated are change in best-corrected visual acuity, change in retinal thickening on OCT, resolution of traction, if present, and surgical complications (See Figures 2 and 4). Eligible subjects should have at least one eye in which vitrectomy is being used to treat DME as seen on clinical examination. Best corrected visual acuity (BCVA) should be a Snellen equivalent of approximately 20/800 or better (ETDRS ≥3 letters); however the acuity in the primary analysis cohort was a Snellen equivalent of 20/63 to 20/400.
Up to 400 subjects will be enrolled, of which approximately 100 subjects will have macular traction, baseline visual acuity between an approximate Snellen equivalent of 20/63 to 20/400, retinal thickness ≥300 µm, and cataract surgery not combined with vitrectomy. Subjects will be treated with vitrectomy per the investigator's "usual routine." Data first will be analyzed at six months, but the final data collection will occur after three years of follow-up. At this time point, long-term visual outcomes and complications such as cataract formation will be analyzed.
• An Observational Study of the Development of Diabetic Macular Edema Following Scatter Laser Photocoagulation. This prospective, multi-center nonrandomized treatment study will determine the incidence and extent of macular edema following scatter panretinal photocoagulation using ocular coherence tomography measurements. The study will also determine if the development of edema varies according to the number of sittings used for the treatment. One-hundred and fifty eyes will be included in the study, and eligible eyes should not have macular edema prior to PRP. The study eye must have a central retinal thickness ≤299 µm on OCT, and early proliferative or severe nonproliferative diabetic retinopathy that indicates performing scatter PRP in one or four sessions.
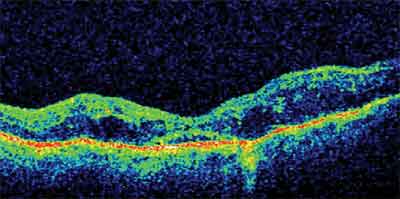
Figure 2. Pre-vitrectomy OCT image of DME with associated vitreomacular traction. Vision was 20/200.
Treatment will be given as 1,200 to 1,600 burns during one sitting, or four sittings separated by four weeks with a total of 1,200 to 1,600 burns. Subjects whose treatment is divided into four sessions will receive 300 burns in each of the first two sittings. The treatment groups will not be randomized. Study participants will be followed for 34 weeks for the development of retinal thickening and change in visual acuity.
• Subclinical Diabetic Macular Edema Study. This is a prospective, multicenter observational study. Its primary objective is to determine the progression of subclinical DME, which is defined as no edema involving the center of the fovea on biomicroscopy, but with a center point thickness on OCT at least two standard deviations beyond a mean value between 225 and 299 µm. Progression of the edema is defined as an increase in the center point thickness of at least 50 µm to >300 µm and/or treatment for DME. Secondary objectives of the study are to evaluate predictive factors for the presence of subclinical DME and to determine the indicators of risk for progression.
Eligible subjects must have a visual acuity of 20/32 or better in the study eye, normal macular thickness on examination not indicating treatment, and a center point thickness of 225 to 299 um. A total of 220 subjects will be followed for two years, with examinations performed at years one and two. At these time points, investigators will determine the presence of the main outcome, which is progression of macular edema as described above, or the development of DME that required treatment.
• A Phase II Evaluation of Anti-VEGF for Diabetic Macular Edema. This study is a Phase II randomized, multicenter clinical trial involving 100 subjects. Its goal is to provide preliminary data on the dose and dose- interval-related effects of intravitreous bevacizumab (Avastin) on retinal thickness and visual acuity in subjects with DME. This study will also provide preliminary data on the safety of intravitreous bevacizumab, and will aid in planning a Phase III anti-VEGF trial.
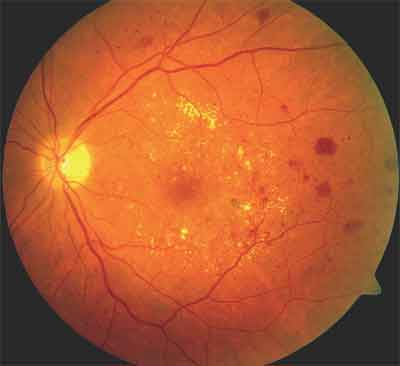
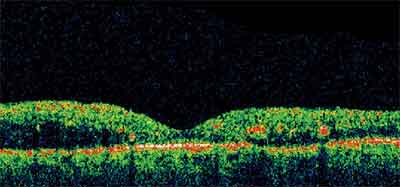
Figure 3. DME with evidence of exudative changes, macular microaneurysms, and retinal thickening.
Studies in Follow-up Phase
• A Pilot Study of Laser Photocoagulation for Diabetic Macular Edema. This is a randomized multi-center clinical trial aiming to compare the ETDRS "standard method" of laser to a "mild macular grid" treatment for DME (See Figure 3). The "mild macular grid" treatment is milder in intensity but more extensive in number of spots as compared to the ETDRS laser protocol. The study eyes have a visual acuity of 20/400 or better, with definite retinal thickening based on clinical examination at or within 500 µm of the macular center that indicates laser treatment. OCT measurements show a retinal thickness of 250 µm or more in the central subfield, or a thickness of 300 µm or more in any one of four subfields adjacent to the central subfield.
A total of 263 subjects with 323 eligible eyes were randomized to receive either modified ETDRS laser photocoagulation or "mild macular grid" photocoagulation. For those subjects with both eyes eligible for the study, one eye was randomized to one treatment technique, while the other eye received the other treatment technique. Subjects are to be followed for three years, with the primary efficacy outcomes analyzed at one year being the change from baseline in visual acuity and in retinal thickening as measured on OCT.
• A Randomized Trial Comparing Intravitreous Triamcinolone Acetonide and Laser Photocoagulation for Diabetic Macular Edema. This randomized, multicenter trial was designed to determine whether intravitreous triamcinolone acetonide (IVT) injections at doses of 1 mg or 4 mg produce greater benefit, with an acceptable safety profile, than macular laser photocoagulation in the treatment of diabetic macular edema. The study also aims to compare the safety and efficacy of the 1-mg and 4-mg doses of IVT. Subjects had a best-corrected visual acuity approximate Snellen equivalent of 20/40 to 20/320 in the study eye. The macular edema involves the central macula as seen on clinical examination and OCT, with a mean retinal thickness on two separate OCT measurements ≥250 µm in the central subfield. The fellow eye was either eligible for enrollment or had a visual acuity ≥20/400, with no history of previous IVT treatment.
The treatment groups were randomly assigned among 813 eyes of 689 subjects. Subjects with one study eye were randomly assigned to one of three treatment groups: laser photocoagulation; 1-mg IVT; or 4-mg IVT. For subjects with two study eyes, the right eye was assigned to one of the three treatment groups, and the left eye received the alternative treatment, with the dose of IVT randomly assigned.
The subjects will be followed for three years, with a preliminary outcome assessment at the end of one year. Both efficacy and safety outcomes will be evaluated. Efficacy is primarily defined as ≥15 letter improvement from baseline at three years, and secondarily as change in retinal thickening as measured on OCT. Safety outcomes that will be evaluated include increased intraocular pressure or glaucoma, cataract development or surgery, endophthalmitis (bacterial or inflammatory), and retinal detachment.
• A Pilot Study of Peribulbar Triamcinolone Acetonide for Diabetic Macular Edema. This Phase II randomized, multicenter clinical trial has four objectives: 1) to estimate the incidence of improvement of DME following a posterior peribulbar injection of 40 mg triamcinolone acetonide compared with laser; 2) to estimate the incidence of improvement of edema following an anterior peribulbar injection of 20 mg of triamcinolone acetonide compared with laser; 3) to estimate the incidence of elevated intraocular pressure with either type of injection; and 4) to compare the incidence of improvement of macular edema with peribulbar triamcinolone alone or followed by laser photocoagulation. Eligible eyes have a visual acuity ≥69 letters, retinal thickening due to DME based on clinical examination, and a retinal thickness of ≥250 µm or more in the central OCT subfield. Per the discretion of the investigator, the subject should not have already been treated with maximal laser, and should have potential for improvement in visual acuity with either peribulbar corticosteroids or laser.
Figure 4. Post-vitrectomy OCT image of the subject in Figure 2. The retinal edema has resolved, and vision has improved to 20/80.
The treatment groups are comprised of a total of 113 subjects with 137 randomized eyes, at least 40 of which have not received prior treatment for diabetic macular edema. Subjects with one study eye were randomized into one of 5 treatment groups: 1) modified ETDRS focal laser photocoagulation; 2) posterior peribulbar injection of 40 mg triamcinolone; 3) anterior peribulbar injection of 20 mg triamcinolone; 4) the posterior triamcinolone injection followed in one month by laser; and 5) the anterior triamcinolone injection followed in one month by laser. For subjects with both eyes enrolled in the study, one eye was randomly assigned to laser and the other eye was randomly assigned to one of the four triamcinolone groups. The subjects are to be followed for three years, with the primary efficacy and safety outcomes at eight months. The primary efficacy outcome is visual acuity and secondary efficacy outcomes include retinal thickening on OCT, persisting or recurring edema indicating retreatment within the first eight months, and a change in the area of retinal thickening with involvement of the central macula based on color photos. Safety outcomes to be monitored include IOP elevation or glaucoma, cataract, ptosis, and complications of the injection procedure.
Completed Studies
• Temporal Variation in Optical Coherence Tomography Measurements of Retinal Thickening in Diabetic Macular Edema. This multicenter observational study involved 107 subjects and occurred on a single day from 7:30 a.m. to 4:30 p.m. Eligible subjects had at least one eye with definite retinal thickening due to DME in the central macula, with retinal thickening of the central subfield ≥225 µm on OCT. Its objective was to determine the proportion of eyes that demonstrate a meaningful change in central retinal thickening measured on OCT throughout the day and to establish a time course of change for eyes that experience diurnal change in central retinal thickening. It also evaluated intra-observer and inter-observer variability on OCT measurements.
Studies Under Consideration
• Intravitreous anti-VEGF or Steroid in Combination with Laser Photocoagulation for Diabetic Macular Edema. The objective of this study currently is planned to be to evaluate the safety and efficacy of intravitreous anti-VEGF treatment or intravitreous corticosteroids, alone or in combination with focal photocoagulation in eyes with center-involved diabetic macular edema.
The subjects will be followed for three years. Both efficacy and safety outcomes will be evaluated.
• Intravitreous anti-VEGF or Steroid as Adjunctive Treatment to Panretinal (Scatter) Photocoagulation. The objective of this study currently is to determine whether either an intravitreous injection of anti-VEGF or an intravitreous injection of corticosteroid can reduce the risk of visual acuity impairment that can occur following panretinal photocoagulation in eyes with evidence of center-involved macular edema that are undergoing PRP for proliferative diabetic retinopathy. Both efficacy and safety outcomes will be evaluated.
All of the above studies require that study participants be ≥18 years old to be eligible for enrollment. Subjects or eye care providers or diabetic care providers who are interested in more information may access the network at the website http://public.drcr.net.
The four central resource centers in the Network are as follows: Office of the Network Chair, Neil M. Bressler, MD (Wilmer Eye Institute at Johns Hopkins University, Baltimore); the Coordinating Center directed by Roy W. Beck MD, PhD (Jaeb Center for Health Research, Tampa, Fla.); the Fundus Photograph Reading Center directed by Ronald P. Danis, MD (University of Wisconsin-Madison, Madison, Wisc.); and the National Eye Institute under program director Paivi Miskala, PhD (Bethesda, Md.). The Barnes Retina Institute at Washington University in St. Louis is a participating site for a number of the protocols described in this article.
Physicians interested in being involved with the DRCR.net may apply at the website http://public.drcr.net.
Dr. Apte is an investigator within the DRCR Network but is writing on behalf of himself, not the Network. Contact him at Washington University School of Medicine, Dept. of Ophthalmology, 660 S. Euclid Ave., Box 8096, St. Louis, Mo. 63110. E-mail: apte@vision.wustl.edu. Dr. Prasad is a fellow at the same site.
1. Diabetic Retinopathy Clinical Research Network. Retrieved June 30, 2006 from the National Eye Institute website: http://public.drcr.net.
2. Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552-563.
3. Early Treatment of Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796-1806.
4. Cunningham ET Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmol 2005;112:1747-57.