There’s an ebb and flow to clinical research in the retina. Some years, the halls of ARVO are full of researchers detailing the results of landmark studies, while other years are quieter, as the studies continue in the background. This year promises to be one of the more productive ones, as several studies of treatments for conditions ranging from age-related macular degeneration to diabetic eye disease are coming to fruition. Here’s a look at the latest data, as well as the findings in other areas of retinal research. (Unless otherwise noted, studies received no commercial support.)
AMD in the Crosshairs
Investigators in the Phase III VIEW 1 and 2 studies of intravitreal aflibercept injection (VEGF Trap-Eye, Regeneron) for wet AMD say the drug appears to remain effective for at least two years. The paper’s lead author is a consultant for Regeneron.
|
Investigators say that the proportion of patients who required frequent injections (defined as at least six) in the follow-up period was lower in both the 2q4 (14 percent) and 2q8 (15.9 percent) groups compared with Rq4 (26.5 percent). In the 25 percent of patients who required the most intense therapy (defined as the greatest number of injections), 2q4 (requiring 6.5 injections on average) and 2q8 (requiring 6.6) needed an average of 1.5 and 1.4 fewer injections compared with Rq4 (eight injections). (The 96-week results appear in Table 1, p. 24) The researchers say the incidence of ocular and systemic adverse events was balanced across treatment groups. The most frequent ocular adverse events (occurring in greater than 10 percent of patients) were conjunctival hemorrhage, eye pain, retinal hemorrhage and reduced VA.6962
Researchers from the landmark Comparison of AMD Treatment Trial have identified factors associated with a minimal gain in visual acuity after a year of treatment with ranibizumab or bevacizumab.
In the trial, the researchers randomly assigned participants to receive ranibizumab or bevacizumab on either a monthly schedule or as needed with monthly evaluation. Masked trained readers evaluated fundus morphology, fluid on optical coherence tomography and retinal thickness.
At one year, the investigators say that older age, larger area of choroidal neovascularization and the presence of retinal pigment epithelium elevation were all associated with worse VA (all p<0.006), less gain in VA (all p<0.02) and a lower percentage of at least a three-line gain (%3LG) (all p<0.04). Better VA at baseline was associated with better VA, less gain in VA, and lower %3LG (all p<0.0001). A lesion classified as predominantly or minimally classic was associated with worse VA than an occult lesion (66 vs. 69 letters, p=0.0003). Presence of a retinal angiomatous proliferative lesion was associated with a greater gain in VA (10 vs. 7 letters, p=0.008) and higher %3LG (OR=1.9, 95-percent CI: 1.2 to 3.1). Presence of geographic atrophy was associated with worse VA (64 vs. 68 letters, p=0.001). Abnormal intraocular pressure was associated with worse VA (p=0.002) and less gain in VA (p=0.03). Eyes having a total foveal thickness in the second quartile (325 to 425 µm) had the best VA (p=0.02) and were most likely to gain at least three lines (p=0.004). In addition, the two-year results from the CATT are expected to be released at this year’s meeting.3681
| ||
|
Fourteen U.S. clinical trial sites recruited 65 patients originally treated in the ANCHOR and MARINA trials (enrolled between March 2003 and September 2004) for further ranibizumab treatment in the HORIZON extension study. In this non-interventional, uncontrolled, cross-sectional study, researchers reevaluated patients’ ETDRS vision and performed a complete exam and retinal imaging (fundus photos, fluorescein angiogram, spectral domain optical coherence tomography, fundus autofluorescence) with analysis by the Doheny Reading Center. They also collected serum for genetic studies.
At this point, the cohort is seven to eight years after initiation of ranibizumab treatment. For the primary endpoint, VA, 37 percent of the original study eyes had a VA of 20/70 or better. The mean VA in study eyes was 20/125. Within the cohort, researchers say subgroups showed favorable outcomes, with good vision (at least 20/40) in 23 percent of eyes, and durable CNV quiescence in 35 percent. In contrast, another group showed poor outcomes; 37 percent of eyes had vision of 20/200 or worse. Ongoing exudative disease activity, defined as evidence of CNV leakage or hemorrhage at a study visit or within the previous six months, was found in 54 percent of study eyes, and 23 percent required ongoing treatment with ranibizumab or other AMD treatments. Evaluation of the non-study fellow eyes showed 51 percent of patients had bilateral exudative AMD, with a fifth of the fellow eyes also receiving current or recent AMD treatments. Six percent of patients were legally blind (20/200 or worse) in both eyes. Genetic analysis of the cohort confirmed a strong association of risk alleles of HTRA1 and CFH.3679
Researchers from Tampa, Fla., and Genentech have analyzed the one-year results from the HARBOR study, which compared 2-mg vs. 0.5-mg ranibizumab in patients with subfoveal CNV secondary to AMD.
In the study, 1,097 patients ages 50 and above were randomized to receive one of the two dosages monthly, or on an as-needed basis, after three loading doses. The researchers were either employees of Genentech or received financial support from the company.
At one year, the mean change from baseline in best-corrected VA for the four treatment groups (mean number of ranibizumab injections) was:
• 0.5 mg monthly, +10.1 letters (11.3 injections);• 2 mg monthly, +9.2 letters (11.2 injections);• 0.5 mg p.r.n., +8.2 letters (7.7 injections);• 2 mg p.r.n., +8.6 letters (6.9 injections).
The proportion of patients who lost fewer than 15 letters from baseline at month 12 was 97.8 percent (0.5 mg monthly), 93.4 percent
(2 mg monthly), 94.5 percent (0.5 mg p.r.n.), and 94.9 percent (2 mg p.r.n.). Mean change from baseline in central foveal thickness was -172 μm (0.5 mg monthly), -163.3 μm (2 mg monthly), -161.2 μm (0.5 mg p.r.n.), and -172.4 μm (2 mg p.r.n.). The physicians say that the incidence of ocular and non-ocular adverse events and serious AEs were consistent with previous ranibizumab trials in AMD and were comparable between groups.
The researchers say that, through month 12, superiority of ranibizumab 2 mg monthly over ranibizumab 0.5 mg monthly wasn’t demonstrated by the mean change from baseline in best-corrected acuity, and the p.r.n. groups didn’t meet the specified noninferiority criteria (a margin of four letters) to ranibizumab 0.5 mg monthly. The percentages of patients who lost fewer than 15 letters at one year compared to their vision at baseline were similar in the 0.5 mg p.r.n. and monthly groups.3677
Diabetic Eye Disease
Investigators from Great Neck, N.Y., have found some differences between 0.5 mg and 1 mg of ranibizumab when used to treat clinically significant diabetic macular edema.
In the investigator-sponsored, prospective, randomized clinical trial, eyes with clinically significant DME secondary to diabetic retinopathy were randomized to one of two groups: 0.5-mg ranibizumab (0.5 mg/0.05 ml), or 1-mg ranibizumab (1 mg/0.1 ml). During the first year, each patient received three monthly injections followed by injections on an every-other-month, as-needed retreatment protocol. During the second year, patients were seen every month and received as-needed monthly injections of drug. Some patients were eligible to crossover to a 2-mg dose after the first year.
A 24-month analysis included 42 eyes of 42 patients (20 eyes in the 0.5-mg group, and 22 eyes in the 1-mg group). The two groups didn’t differ at baseline in age, sex, ETDRS letters, OCT central thickness/volume or blood pressure. The 1-mg group had a baseline ETDRS vision of 60.8 letters with a final vision of 71.2 letters at two years (p=0.0001). The 0.5-mg group had a baseline ETDRS vision of 57.5 letters with a final vision of 64.5 letters at two years (p=0.0001). There was a significant difference between the two dose groups across time for ETDRS vision (p=0.035). The 1-mg group had an average gain of 10.4 ETDRS letters as compared with seven letters in the 0.5-mg group (p=0.31). Mean decrease in central foveal thickness was 192 µm in the 1-mg group and 216 µm in the 0.5-mg group (p=0.87). There was a statistically significant difference between the groups with respect to final macular volume at two years, with the 0.5-mg group having a higher volume than the 1-mg group (7.95 vs. 6.85, p=0.027). In the 1-mg group, 27 percent of patients gained 15 or more ETDRS letters, compared to 15 percent of patients in the 0.5-mg group (p=0.46). The average number of injections in the 1-mg group was 12, as compared to 12.9 in the 0.5-mg group (p=0.54). There was no change in blood pressure in either group.1335
Researchers from the University of Wisconsin-Madison and Genentech say that intravitreal ranibizumab shows long-term efficacy for patients with diabetic retinopathy.
In two double-masked, sham-controlled, multicenter trials (RISE and RIDE), researchers randomized 759 diabetic macular edema patients to receive monthly 0.3-mg or 0.5-mg ranibizumab or sham injections. Fundus photos taken at baseline and at specific intervals were graded by a central reading center, and the physicians performed clinical exams monthly. Outcomes of these secondary and
exploratory analyses included analysis of any ≥2- and ≥3-step changes on the ETDRS Severity Scale and a composite clinical progression outcome, including photographic changes plus clinical events (e.g., vitreous hemorrhage or the need for panretinal laser). They note that the ≥2- and ≥3- step worsening on the ETDRS severity scale for eyes are factors associated with increased risk of vision loss.
At two years, the investigators say that ≥2- or ≥3-step DR progression (worsening) was significantly lower and ≥2- or ≥3-step regression (improvement) was significantly higher in ranibizumab-treated eyes vs. sham. Over two years, 33.8 percent of sham-treated eyes experienced clinical progression of DR, compared to 11.2 to 11.5 percent of ranibizumab-treated eyes.1336
Investigators from the Diabetic Retinopathy Clinical Research Network say that the presence of center-involved diabetic macular edema shouldn’t preclude cataract surgery in patients who need it.
In the prospective, non-comparative, observational study, 29 sites enrolled eyes with CI-DME (central subfield thickness of at least 250 μm on time domain or at least 310 μm on spectral domain OCT) that were scheduled to undergo cataract surgery within 28 days of enrollment. VA and OCT outcomes were assessed 16 weeks after cataract surgery. Treatment for DME before, the day of, or after surgery was at the investigator’s discretion.
Sixty of the 63 enrolled eyes (95 percent) completed the 16-week visit.
The patients’ median preoperative electronic ETDRS VA letter score was 59 (~20/63 Snellen equivalent), while the median CSF was 309 μm (based on TD-OCT in 58 eyes). Twenty eyes (33 percent) didn’t receive any pre-, peri-, or post-surgical treatment for DME. Twenty-six eyes (43 percent) received post-surgical treatment at a minimum, the most common of which was intravitreal anti-VEGF injection (73 percent). At 16 weeks, the mean change in VA letter score was +12 (95-percent confidence interval: +8 to +16), and 42 percent (95-percent CI: 29 to 55 percent) were 20/40 or better (read at least 73 letters). Thirty-six eyes (60 percent, 95-percent CI: 47 to 73 percent) had VA improvement of at least two lines (at least 10 letters), while six eyes (10 percent, 95-percent CI: 2 to 18 percent) lost at least two lines (at least 10 letters) of VA. Mean change in CSF was +18 µm (95-percent CI: -18 to +54) relative to the preoperative visit. Thickness increased by at least 1 logOCT unit in 18 percent (95-percent CI: 8 to 28 percent), decreased by at least 1 logOCT unit in 13 percent and resolved in 10 percent.
The researchers say that, even with the presence of CI-DME immediately preceding cataract surgery and standard pre-, peri-, and postop management of macular edema, patients’ VA appears to improve in the majority of eyes by 16 weeks postop.5280
Surgeons from the University of California, Irvine have found the Navilas photocoagulation system to be safe and well-tolerated in proliferative diabetic retinopathy.
Their study was an interventional case series to evaluate the comfort and clinical efficacy of the new panretinal Navilas photocoagulation system in cases of high-risk PDR. In it, 30 consecutive eyes of 30 patients were treated with the Navilas system, which uses a 532-nm laser. The researchers assessed spot size, ablated area, uniformity of the laser spots, duration of treatment and patient comfort during the procedure.
In newly diagnosed PDR, eyes received an average of 1,590 laser spots (range: 1,203 to 1,731) in four quadrants in a single session. The average power intensity used was 258 mW with a 30 ms pulse duration using a 300 µm spot size. The physicians report that laser uptake was consistent across the quadrants; the average procedure time was seven minutes when using the pattern-scan mode and that visibility of the retina was superior under infrared mode in eyes with vitreous hemorrhage. There were no complications during or after the laser treatment. After one to two treatments, the researchers noted regression of neovascularization in 100 percent of patients within three months of the initial treatment.367
Investigators in the READ 3 Research Group say that, at six months, it appears that 2 mg of ranibizumab yields no advantage over a 0.5-mg dose. READ 3 is a Phase II, randomized clinical trial evaluating the two doses in eyes with center-involved DME.
The researchers randomized 152 eyes to either 2-mg or 0.5-mg ranibizumab given monthly for six months. The primary endpoint of the study was the mean change in best-corrected acuity from baseline to month six. After six months, eyes were evaluated monthly and the physicians administered additional ranibizumab on an as-needed basis if DME was still present on OCT.
At baseline, the mean BCVA Snellen equivalent was 20/63 in the 2-mg ranibizumab group and 20/80 in the 0.5-mg group. OCT central subfield thickness was 432 μm in the 2-mg group and 441 μm in the 0.5-mg group. At month six, the primary endpoint of the study, mean change in BCVA was +7.46 ETDRS letters read in 2-mg ranibizumab group and +8.69 letters in the 0.5-mg ranibizumab group. The CSF was reduced by -163.86 μm for the 2-mg group and -169.27 μm for the 0.5-mg group. There were no serious ocular or systemic adverse events related to 2-mg or 0.5-mg ranibizumab.
The researchers say additional studies and long-term analyses of the READ 3 study are needed to determine the potential role of 2-mg ranibizumab in eyes with DME.5282
Retinitis Pigmentosa
An open-label, multicenter Phase Ib study sponsored by QLT has found some potential in the cis-retinoid prodrug QLT091001 for the treatment of early-onset retinitis pigmentosa due to mutations in the RPE-specific protein 65kDa (RPE65) or lecithin retinol acyltransferase (LRAT).
In the study, 17 subjects (age range: 6 to 55 years) received a single, seven-day 40 mg/m2/day course of QLT091001. The researchers assessed Goldmann visual fields and best-corrected VA at baseline and days seven, 14 and 30.
After treatment, average field area improved from baseline by 22 percent (p=0.030) at day seven (statistically significant), 16 percent at day 14 (p=0.13), and 18 percent at day 30 (p=0.096). In an evaluable subject subset (n=14, meeting VF criteria), average VF area improved from baseline by 34 percent at day seven (p=0.005, statistically significant), 29 percent at day 14 (p=0.02, statistically significant), and 23 percent at day 30 (p=0.07). Best-corrected acuity improved by at least five letters in at least one eye in nine subjects (53 percent).
The researchers say that on fMRI (n=2), several areas of the visual and parietal cerebral cortex showed activation after treatment. They add that, within days of the first dose, dark-adapted perimetry performed in a small subset of patients (n=2) showed increases in sensitivity averaging 12 dB at 10 to 50 percent of loci in each eye. The investigators say that full-field sensitivity testing and pupillometry results supported the large increases in sensitivity upon extended dark adaptation, and that QLT091001 showed an acceptable safety profile. Overall, the researchers say that the data supports the expectation that the prodrug bypasses the biochemical blockade of the visual cycle in all retinal areas with viable photoreceptors and RPE.6965
Other Research
Investigators in the randomized, double-masked, Phase III COPERNICUS study say that intravitreal aflibercept injection is showing good efficacy in treating macular edema secondary to central retinal vein occlusion. Several of the researchers are consultants for or employees of Regeneron and/or Bayer HealthCare, the drug’s backers.
|
Two centers in Germany say that the 0.7 mg sustained-release dexamethasone implant Ozurdex (Allergan) is effective in controlling intraocular inflammation in patients with noninfectious posterior/intermediate uveitis. The lead author is a consultant for Allergan.
Fifty eight eyes of 48 consecutive patients were treated with a total of 63 dexamethasone implants. Patients presented with unilateral (n=13) or bilateral posterior/intermediate uveitis (n=45). The researchers say all eyes showed clinical and morphological evidence of decreased inflammation following implant placement. They report that maximum clearance of vitreous haze could be achieved within four weeks in 82 percent of eyes (p<0.01), whereas significant reduction (p<0.05) of central retinal thickness was reached at three months. Within the mean follow-up of 8.8 months, increased IOP (+5 mmHg) was observed in 43 percent of eyes and reached 35 mmHg or above in 12 percent. The investigators say that all events of ocular hypertension could be controlled with conservative treatment. There were no serious ocular or systemic adverse events during the follow-up period.2249
An investigator from the Thrombogenics-sponsored MIVI-TRUST Phase III study says that the company’s drug, ocriplasmin, is well-tolerated and could be a good approach for treating vitreomacular traction.
The MIVI-TRUST program consisted of two large Phase III clinical trials designed to compare the results of a single intravitreal injection of 125 µg (100 µl) of ocriplasmin with a single 100-µl placebo injection for the pharmacological treatment of symptomatic vitreomacular adhesion. The primary endpoint was the nonsurgical resolution of VMA at day 28. Researchers followed the patients for six months. Out of a total of 652 treated eyes, 266 had VMT (without concurrent macular hole and/or epiretinal membrane) at baseline. Of these, 188 received a single 125-µg (100 µl) intravitreal injection of ocriplasmin and 78 eyes received 100 µl of intravitreal placebo injection. Assessments included best corrected VA, OCT and a review of adverse events.
At day 28, 29.8 percent of ocriplasmin-treated eyes with VMT at baseline had resolution of VMA on OCT, compared to 7.7 percent of placebo-treated eyes (p≤0.001). In the ocriplasmin-treated patients with resolution of VMA, VA improvements of at least two lines were achieved in 41.1 percent and at least three lines in 14.3 percent at month six. Ocular adverse events were generally mild and included floaters and photopsia, and researchers say most adverse events occurred within the first seven days of treatment. For example, the incidence of floaters was 12.9 percent in the first week and 3.9 percent between one week and the study’s end at six months. There were no cases of endophthalmitis.2754
|
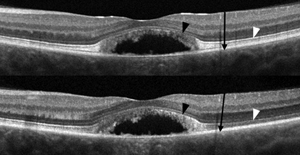
The researchers say that SD-OCT images acquired through multiple pupil entry positions using the eOCT technique show marked variation in the reflectivity of specific retinal layers. In some eyes containing macular pathology there is attenuation of the second and third hyper-reflective outer retinal bands (PR bands), which they assert may be interpreted clinically as photoreceptor loss. They say this appearance may be due to the alteration of photoreceptor orientation, which emulates the expected directional reflectivity observed in eOCT images of normal subjects.
While maintaining central fixation, the team acquired frame-averaged B-scans with the SD-OCT beam passing through the optical center and then through eccentric pupil positions in 32 normal subjects using Cirrus HD-OCT. The researchers registered, normalized and manually segmented the eOCT image sets to demarcate several retinal regions, one containing the PR bands. They calculated the total pixel values within each region from each image in the set. They also performed adaptive optics scanning laser ophthalmoscopy and eOCT imaging in patients with Best’s disease and AMD.
In normal subjects, the investigators say reflectivity from the PR bands was uniformly maximal through the optical center and demonstrated a high degree of variance (CV=24 percent) compared to the inner retina (CV=5 percent) and the true outer nuclear layer (CV=4 percent) in eOCT image sets. Images of a 20/40 patient in the pseudo-hypopyon stage of Best’s disease acquired through the optical center demonstrated a severely attenuated appearance of the parafoveal second hyper-reflective outer retinal band. However, when the eye was imaged eccentrically, this band was actually revealed to be intact (See image, above). Registered AOSLO images verified the presence of an unambiguous cone mosaic within these locations.
The researchers say the study confirms that pupil position through which SD-OCT images are acquired and the presence of retina-deforming pathology can alter the appearance of the PR bands, and that eOCT can enhance the clinical assessment of the photoreceptors.3169 REVIEW
Dr. Regillo is a professor of ophthalmology at Thomas Jefferson University and director of the Retina Service at the Wills Eye Institute.