The Impact of AREDS
In 2001, the researchers of the Age-Related Eye Disease Study reported their landmark findings that patients with dry AMD who took a combination of antioxidants and zinc experienced a decrease in disease progression.1 The study divided the patients based on severity of their AMD: mild disease (category 1); intermediate disease (categories 2 and 3); and advanced disease (category 4). The primary endpoints of the AREDS trial were progression to advanced AMD and the degree of vision loss.
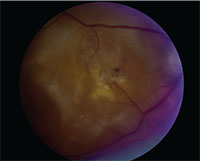 |
| Central disciform scar in a patient with exudative AMD. |
After analyzing the results, the study’s physicians explicitly recommended vitamin supplementation only in patients with either intermediate disease or advanced disease in only one eye. There was a clear benefit in these patients, as they demonstrated decreased visual loss and decreased progression to more advanced stages of AMD. The results of the study showed no clear benefit in the patients with category 1 disease (those with only very small drusen in the macula).
These results recommend vitamins to all but two large cohorts of patients, the first of which is patients with category 1 disease. Even in a 10-year follow-up study with patients from the original AREDS report, there was no significant difference in the progression of disease in category 1 patients receiving placebo versus therapy, confirming the original study’s position of not recommending vitamin use to patients with only mild disease.2
The second group of patients for which vitamin use isn’t recommended is those with significantly advanced disease that severely affects not just one eye, but both of them. However, many studies have shown that these two groups of patients are actually taking AREDS vitamins.3,4
For a variety of reasons, such as seeing a television commercial, as many as 68 percent of these patients for whom there are no recommendations to take AREDS vitamins are actually taking them,3 a rate of vitamin use similar to the 71 percent adherence rate reported in the AREDS study. Interestingly, most of these patients report being told by their ophthalmologists to take the vitamin supplementation, despite no formal AREDS recommendation.
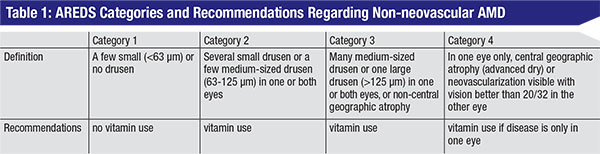 |
In another recent survey of 64 patients with AMD, only 59 percent of them reported that they were taking a vitamin of any kind. Of those patients eligible for AREDS vitamin supplementation, 75 percent reported that no vitamin use had been recommended to them.4 These studies are dependent on patient responses, which is a big limitation, but the results are still noteworthy.
Can We Do More?
Ophthalmologists and retina specialists are often asked by patients with very mild or very severe disease if they should be taking the vitamins, or, conversely, they are faced with a decision to stop the vitamins in those categories of patients.
Patients with bilateral neovascular AMD can at least turn to anti-vascular endothelial growth factor therapies.5-7 Still, the questions linger: Why not take an additional vitamin that may possibly reduce vision loss? Would the addition of antioxidants to anti-VEGF therapy further prevent vision loss? One recent study, partially sponsored by a then-subsidiary of Bausch + Lomb (which makes supplements), reported that it might be possible. In the small study (40 patients divided into four groups), researchers reported that anti-VEGF therapy in addition to omega-3 fatty acid supplementation may be superior to anti-VEGF therapy alone in terms of lowering intravascular VEGF levels, but added that the effect on CNV levels and vision still need to be elucidated.8
It’s unlikely that any analysis will be performed in an effort to investigate the use of AREDS vitamins alone in patients with bilateral neovascular AMD due to the advent of anti-VEGF therapy. However, it could be worthwhile for future studies to evaluate the degree of vision loss in patients with bilateral wet AMD taking both AREDS vitamins and receiving anti-VEGF versus the outcomes in patients only receiving anti-VEGF therapy.
Despite the AREDS trial not recommending therapy for patients with advanced disease per their primary endpoints, one of the secondary endpoints was tracking visual acuity loss in the eyes with baseline neovascular AMD (the non-study eyes). They divided these eyes into two groups, one with a baseline vision of 20/100 or better (260 eyes) and another with a visual acuity of 20/200 or better (352 eyes). It’s difficult to follow patients with neovascular AMD using standard outcome measures such as visual acuity since the disease is already advanced, but the study data concluded that there was a significant difference between the treatment groups and the placebo groups when comparing progression of visual loss. Specifically, there was a significant difference for the patients with a better baseline visual acuity who received antioxidants vs. placebo (OR 0.35; 99% CI, 0.15-0.81).1 This was the only group with a significant p-value and a confidence interval that didn’t cross 1.0. Other arms saw a significant p-value: the eyes with a VA of 20/200 who received antioxidants, and both groups that received antioxidants plus zinc, but all of these cohorts had a CI that crossed 1.0. Table 2 highlights this secondary endpoint and the differences between the patient groups.
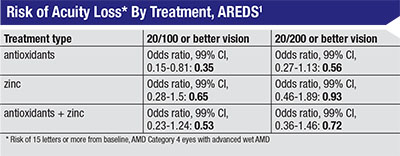 |
Still, there is the group of patients with bilateral central geographic atrophy that wasn’t addressed by the AREDS report. Unfortunately, no separate analysis could be performed in the central geographic atrophy patients due to the limited number of patients with this disease at baseline in the AREDS trial and, to date, there are no published studies examining the effect of AREDS vitamins on patients with bilateral central geographic atrophy.
It’s also worth noting that as more information emerges regarding genetics and the role that specific genotypes play in the pathophysiology behind AMD,9 most research in the literature hasn’t shown better outcomes between certain genotypes in response to nutritional supplementation.10,11 Only one study showed a difference in patients with the CFH genotype,12 but this benefit wasn’t duplicated by the AREDS group. AREDS Report Number 38 found no difference in the progression of disease in patients with intermediate disease at the beginning of the study based on genotype. Previous studies had suggested that genetic testing be done before starting patients with AMD on nutritional supplementation,11 but the AREDS report suggests that it’s not currently beneficial for genetic analysis to be done, as there was no benefit in patients with specific genotypes over other genotypes. It bears mentioning that this report didn’t analyze patients with bilateral severe disease or mild disease and instead only assessed progression of AMD in patients with category 3 or 4 disease.
Diet and AMD
Other studies have examined the role of various diets and vitamins and their effect on the progression of AMD.13,14 One study found that adherence to a Mediterranean diet might be beneficial for certain patients. The Mediterranean diet is plant-based, and consists of fruits, vegetables, whole grains, legumes and nuts, with an allowance for moderate consumption of fish and wine. It replaces butter with healthier oils such as olive oil or canola oil, and uses herbs instead of salt to flavor foods. Specifically, the study’s researchers found decreased progression in AMD in patients with the CFH genotype, but not in patients homozygous for the risk genotype of CC.15 This study is interesting because it also found no effect of AREDS supplementation in slowing disease progression in those patients with the risk genotype. Neither the Mediterranean diet nor AREDS supplementation slowed the progression of disease in these patients. It’s also important to note that this study didn’t use the AREDS classification scheme, nor did it include patients with bilateral advanced disease at the beginning of the study. Still, it’s worth mentioning to patients, even those with mild disease, that dietary changes may slow disease progression, though those AMD patients with high-risk genotypes may not benefit from changes in their diet or from the addition of vitamin supplementation.
Looking Ahead
Many patients with AMD who are eligible to receive vitamins under the AREDS guidelines aren’t receiving appropriate vitamin supplementation and, according to some surveys, it’s because physicians are not appropriately recommending vitamin use. It’s extremely important that physicians recommend vitamins to the appropriate, AREDS-eligible patients. We also encourage providers to look specifically at their patients who aren’t eligible for vitamin supplementation under AREDS guidelines and perhaps reconsider supplementation for them. This is especially true for those with bilateral wet AMD, since secondary endpoints from the AREDS trial show decreased vision loss when supplemented with AREDS vitamins, and other studies show decreased intraocular VEGF levels in patients in whom anti-VEGF therapy and AREDS vitamins are used in combination.8
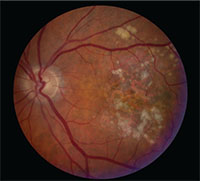 |
| Central geographic atrophy in a patient with macular degeneration. |
Finally, dietary modification may play a prominent role in limiting disease progression for many patients with AMD, including those who only have a mild level of the disease, though certain genotypes may still progress towards advanced disease despite dietary and medication adjustments. REVIEW
Dr. Starr is an ophthalmology resident at Mayo Clinic in Rochester, where Dr. Bakri is a professor of ophthalmology and director of the vitreoretinal surgical fellowship. Correspondence should be directed to Dr. Bakri at bakri.sophie@mayo.edu. This work was supported in part by an unrestricted grant from Research to Prevent Blindness (New York, N.Y.).
1. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417–1436.
2. Chew EY, Clemons TE, Agron E, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol 2014;132:272-277.
3. Yu AL, Paul T, Schaumberger M, Welge-Lussen U. Factors affecting the use of antioxidant supplements in patients with late AMD. Clin Ophthalmol 2014;8:1227–1232.
4. Hochstetler BS, Scott IU, Kunselman AR, Thompson K, Zerfoss E. Adherence to recommendations of the age-related eye disease study in patients with age-related macular degeneration. Retina 2010;30:1166–1170.
5. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355:1432–1444.
6. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2011;364:1897–1908.
7. Rosenfeld, PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355:1419–1431.
8. Rezende, FA, Lapalme E, Qian CX, et al. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am J Ophthalmol 2014;158:1071–1078.
9. Fritsche LG, Chen W, Schu M, et al. Seven new loci associated with age-related macular degeneration. Nat Genet 2013;45:433–439:439e1–2.
10. Chew EY, Klein ML, Clemons TE, et al. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS Report Number 38. Ophthalmology 2014;121:2173–2180.
11. Klein, ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology 2008;115:1019–1025.
12. Awh, CC, Lane AM, Hawken S, Zanke B, Kim IK. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology 2013;120:2317–2323.
13. Krishnadev N, Meleth AD, Chew EY. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol 2010;21:184–189.
14. Merle BM, Silver RE, Rosner B, Seddon JM. Dietary folate, B vitamins, genetic susceptibility and progression to advanced nonexudative age-related macular degeneration with geographic atrophy: A prospective cohort study. Am J Clin Nutr 2016;103:1135–1144.
15. Merle BM, Silver RE, Rosner B, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr 2015;102:1196–1206.
16. Hanus J, Zhao F, Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol 2016;100:122–127.
17. Schmidl D, Garhöfer G, Schmetterer L. Nutritional supplements in age-related macular degeneration. Acta Ophthalmol 2015;93:105–121.
18. Zucchiatti I, Parodi MB, Pierro L, et al. Macular ganglion cell complex and retinal nerve fiber layer comparison in different stages of age-related macular degeneration. Am J Ophthalmol 2015;160:602–607.



