Intravitreal anti-vascular endothelial growth factor agents are the standard of care for diabetic macular edema, and perhaps even diabetic retinopathy in general. However, despite their proven efficacy, anti-VEGF injections aren’t curative, and many patients require long-term therapy with regular injections. Since DR primarily affects working-age individuals; for a 21-year-old college student or a working mother, regular injections might not be tenable and could reduce compliance.1 These patients already face a heavy burden from managing their diabetes in general. Help appears to be on the way, however. In this review, we discuss the current efforts to improve the longevity and durability of existing anti-VEGF agents, while keeping an eye on alternative agents in the pipeline that might eventually join the clinician’s armamentarium.
High-dose Aflibercept (Eylea)
There is excellent Phase III data supporting the use of aflibercept for both DME and DR. Aflibercept binds all isomers of VEGF-A, but also binds VEGF-B and placental growth factor. The VIVID and VISTA trials reported improvement in visual acuity and macular thickness in eyes with DME.2 In DRCR.net’s Protocol T, aflibercept was compared to ranibizumab and bevacizumab for DME.3 All drugs improved vision at one year: aflibercept (+13 letters); ranibizumab (+11 letters); and bevacizumab (+10 letters). However, patients with more significant disease at baseline (worse than 20/50 vision or >400 µm macular thickness), fared better with aflibercept.
Post-hoc analyses of VIVID and VISTA data suggest stabilization or even improvement of retinal non-perfusion and diabetic retinopathy, prompting further study.4 The Phase III PANORAMA trial found eyes with moderately-severe to severe non-proliferative diabetic retinopathy had significant improvement in the degree of retinopathy following aflibercept treatment, either q8 weeks or q16 weeks.5 The DRCR.net Protocol W is a similar Phase III trial, with results expected in 2022.
If the standard 2-mg aflibercept dose is effective for DME, could an 8-mg dose have increased efficacy and durability? To answer this question, researchers have started the PHOTON study (NCT04429503), a Phase II/III, randomized, double-masked study comparing the efficacy and safety of 8 mg high-dose aflibercept with the current FDA-approved dose of 2 mg aflibercept.5 The primary objective of the study, which is currently recruiting patients, is to determine if treatment with high-dose aflibercept at intervals of 12 or 16 weeks provides non-inferior best corrected visual acuity (BCVA) compared to aflibercept dosed every eight weeks. The study aims to enroll 640 patients with a slated end date of April 2022. Depending on the results of PHOTON, high-dose aflibercept could build a bridge from the current anti-VEGF monotherapy regimens to more durable treatments in the future.
Brolucizumab (Novartis)
At a size of ~26 kDa, the humanized single-chain antibody fragment brolucizumab may provide enhanced tissue penetration, clearance and drug delivery characteristics compared to more traditional “bulky” anti-VEGF agents. By comparison, ranibizumab and aflibercept have molecular weights of 48 kDa and 115 kDa respectively.6 The molar dose of brolucizumab is 11.2 to 13.3 times higher than that of aflibercept, permitting greater drug concentrations and therefore longer duration. Brolucizumab achieved positive results in two Phase III trials for wet AMD, HARRIER (NCT02434328) and HAWK (NCT02307682), leading to its FDA approval in October 2019 for that disease.7
As with ranibizumab and aflibercept, drugs demonstrating efficacy for wet AMD are often evaluated for DR/DMR. Three current Phase III studies evaluating brolucizumab for DR/DME are KITE, KESTREL and KINGFISHER.
The first Phase III trial, KITE, achieved positive topline results comparing brolucizumab 6 mg to aflibercept in DME.8 According to the company, the trial met its primary and key secondary endpoints, demonstrating non-inferiority for brolucizumab versus aflibercept 2 mg in mean change in best-corrected visual acuity at week 52.
Similarly, the KESTREL study was started as another randomized, double-masked, noninferiority study in DME patients.9 The experimental arms compare 3 mg and 6 mg doses of brolucizumab given every six weeks for five injections, followed by maintenance injections every eight or 12 weeks until the end of the study. The comparator arm is 2-mg aflibercept dosed every four weeks for five injections and then every eight weeks as maintenance until completion of the study. KESTREL reached full enrollment with 571 patients in March 2020. Study completion is expected in 2021.
KINGFISHER is randomly assigning participants with DME to receive 6 mg of brolucizumab every four weeks or 2 mg of aflibercept every four weeks.10 The primary outcome is change in BCVA from baseline to 12 months. Data from the full enrollment of 521 patients in KINGFISHER are expected in 2021.
Despite the efficacy of brolucizumab for wet AMD, and its superior pharmacokinetics, many retina specialists are concerned about the risk of occlusive vasculitis and blindness with the drug. In June 2020, the FDA approved an updated brolucizumab label that includes additional safety information specifically including the characterization of adverse events, retinal vasculitis and retinal vascular occlusion. These effects were noted as part of the spectrum of intraocular inflammation observed in HAWK and HARRIER for AMD.11,12 Entering 2021, pending the results of KESTREL and KINGFISHER, it’s unclear whether these adverse events will outweigh the potential benefits for brolucizumab.
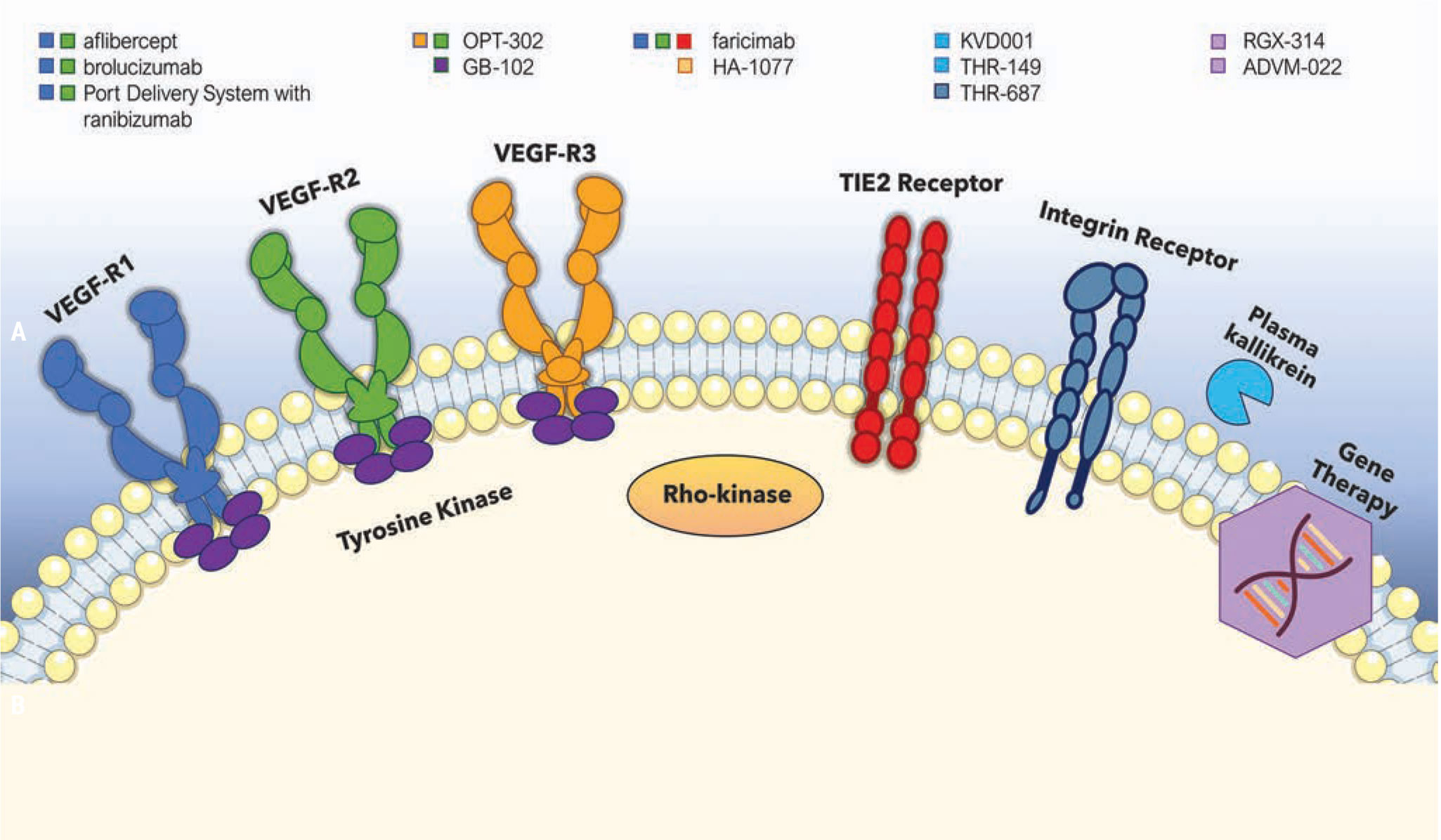 |
|
Several current therapeutic agents and their specific cellular targets. |
Faricimab (Genentech/Roche)
Faricimab is the first bispecific monoclonal antibody designed for intraocular use. With two arms, the antibody independently binds and neutralizes both VEGF-A and angiopoietin-2 (Ang-2); this synergistically promotes vascular stability.13 Inhibition of the VEGF pathway has long been exploited in DR, but targeting the Ang-2/Tie pathway is of recent therapeutic interest. Ang-1 and Ang-2 are key cytokines in the angiopoietin pathway that interact with transmembrane receptor tyrosine kinase (Tie-2). In healthy states, Tie-2 is bound by angiopoietin-1, which is a protective factor, promoting vascular stability, pericyte recruitment and the inhibition of vascular permeability factors. However, in angiogenic states, the competitive inhibitor angiopoietin-2 is upregulated, displacing Ang-1, and causing endothelial destabilization, inflammation and breakdown of the blood-retina barrier. The hope is that Ang-2 blockade may further stabilize vasculature structures in patients with DME.
The Phase II BOULEVARD study14 evaluated the efficacy and durability of faricimab in patients with DME compared to monthly ranibizumab. In the study, 229 anti-VEGF treatment-naïve patients with center-involving DME were randomized into one of three arms. Participants received 1.5-mg faricimab, 6-mg faricimab or 0.3-mg ranibizumab every fourth week up to week 20, after which all subjects underwent an observational period until week 36. At week 24, the patients in the faricimab 6-mg arm had a mean improvement in visual acuity of 13.9 letters compared with 10.3 letters for the ranibizumab arm. Faricimab-treated patients also showed anatomic improvements at week 24 compared with ranibizumab- treated patients, namely a reduction in central subfoveal thickness and improvements in DR severity. These data from BOULEVARD suggested a benefit of combined Ang-2/VEGF-A blockade compared to anti-VEGF monotherapy.
Based on these promising Phase II results, two identical Phase III trials, YOSEMITE (NCT03622580) and RHINE (NCT03622593) were initiated to further evaluate the safety and efficacy of faricimab.15,16 In each study, more than 900 patients worldwide were randomized to one of three arms. One arm received faricimab 6 mg every eight weeks. The second arm was a personalized treatment interval arm in which faricimab 6 mg was spread to every 16 weeks as long as the patient’s central subfoveal thickness didn’t increase and require more frequent treatment. The comparison arm was 2-mg aflibercept dosed every four weeks for 16 weeks, then every eight weeks thereafter.
In December 2020, the study met the primary endpoint of non-inferiority to aflibercept. The long-term safety and tolerability of faricimab is being evaluated in the Phase III Rhone-X study (NCT04432831), with an estimated completion date of August 2023.
Phase III data from YOSEMITE and RHINE were presented in February 2021 at the Angiogenesis, Exudation, and Degeneration 2021 conference.18 In YOSEMITE, the average vision gains from baseline were 11.6 and 10.7 eye-chart letters in the faricimab personalized-treatment arm and two-month arms, respectively. The aflibercept arm reported vision gains of 10.9 letters. In the identical RHINE trial, the average vision gains from baseline were 10.8 and 11.8 letters in the faricimab personalized-treatment interval arm and two-month arms, respectively, and 10.3 letters in the aflibercept arm. As with other monoclonal antibodies, some patients developed inflammation after faricimab treatment.
PDS with Ranibizumab (Genentech/Roche)
Ranibizumab was the first FDA approved anti-VEGF agent for both DME and DR. There’s strong data from the RIDE and RISE studies demonstrating the efficacy of ranibizumab for DME. Post-hoc analyses of RIDE and RIDE19 recognized the benefit of anti-VEGF blockage in improving the degree of DR and retinal nonperfusion. This led to the DRCR.net protocol S, which found ranibizumab to be non-inferior to PRP for proliferative diabetic retinopathy, with reduced risk of center-involving DME in ranibizumab-treated eyes.20
Could a longer-acting ranibizumab, delivered via a surgically implanted depot, allow for long-term treatment of DR and DME while reducing the need for regular injections? The Port Delivery System with Ranibizumab allows continuous release of ranibizumab into the vitreous via passive diffusion, and is intended to reduce the frequency of intravitreal injections, potentially allowing patients with DME to go several months before needing a refill of the implant.21 The device is self-sealing and requires surgical implantation; it can be refilled in the office via injection through the conjunctiva. At the moment, the PDS holds 20 µl of a customized formulation of ranibizumab (100 mg/ml). This dosage was found to be the most effective dose in the Phase II LADDER trial in wet AMD, looking at visual and anatomic success.22
For DME, the Phase III noninferiority study PAGODA has been started.23 In total, 550 patients with DME were randomized to receive the PDS 100 mg/ml, refilled at fixed six-month intervals, or monthly intravitreal injections of ranibizumab 0.5 mg. The primary endpoint is change in BCVA from baseline to week 64. Genentech is expected to release primary outcome data in 2021.
By contrast, PAVILION (NCT04503551) was started to evaluate the efficacy, safety and pharmacokinetics of PDS for the treatment of DR in patients without DME.24 The exclusion of DME could provide interesting data to compare with PAGODA. Patients with moderately severe or severe nonproliferative DR are randomized to receive PDS with ranibizumab 100 mg/ml or 0.5-mg ranibizumab injections. The primary endpoint is the percentage of participants with an improvement of more than two steps from baseline on the ETDRS Diabetic Retinopathy Severity Scale at one year. Participants will receive two intravitreal 0.5-mg ranibizumab injections before PDS insertion, and then the PDS will be refilled with 100 mg/ml ranibizumab every 36 weeks. A comparator arm will undergo regular examinations every four weeks until crossing over to receive the PDS implant. PAVILION is actively recruiting, aiming for 160 patients.
The concept of a surgically implanted drug depot is intriguing but does carry potential risks. The LADDER study found that 10.6 percent of patients developed vitreous hemorrhage, 2.8 percent developed retinal detachment, 1.7 percent developed endophthalmitis and 15.1 percent developed cataract.25 Retinal surgeons and patients must weigh these potential risks against the potential benefits.
RGX-314 Gene Therapy
Gene therapy has shown promise for the treatment of inherited retinal diseases, and recently there’s been a push to find gene therapy solutions for AMD and DR. RegenxBio has been at the forefront of this development with its novel gene therapy, RGX-314, a vector designed to bind and neutralize VEGF in a manner similar to ranibizumab.26 RGX-314 utilizes adeno-associated virus serotype 8 (AAV8) as its vector, with research suggesting that AAV vectors provide long-term transgene expression.27 The gene therapy vector is preferentially taken up by retinal cells, leading to high levels of production of the monoclonal antibody fragment. Interestingly, the company is advancing two separate routes of ocular administration of RGX-314: a one-time subretinal administration during vitrectomy; and in-office suprachoroidal delivery. The hope is that the long-standing and stable production of the anti-VEGF therapeutic protein will reduce the need for frequent intravitreal injections.
In December 2020, RegenxBio announced that the first patient had been dosed in ALTITUDE, a Phase II trial designed to evaluate the suprachoroidal space (SCS) delivery approach with RGX-314 using the SCS Microinjector, for the treatment of DR without DME.28 The trial is expected to enroll 40 patients with DR across two cohorts. Patients will be randomized to receive RGX-314 versus observational control at a 3:1 ratio; two dose levels of RGX-314 will be evaluated. Patients won’t receive prophylactic immune-suppressive corticosteroid therapy before or after administration of RGX-314. The primary study endpoint is the proportion of patients with improved DR severity at 48 weeks. Safety and development of DR-related ocular complications are other endpoints that will be evaluated. Initial data from ALTITUDE are expected in 2021.
ALTITUDE comes on the heels of positive one-year data for RGX-314 for wet AMD. In these Phase I and IIa trials, the company reported stable-to-improved visual acuity and retinal thickness, as well as a meaningful reduction in anti-VEGF injection burden, with higher dose levels of RGX-314 at one year.29 With these interim results, and pending the data from ALTITUDE, one-time treatment with anti-VEGF gene therapy may have a meaningful impact on DR patients requiring frequent anti-VEGF, and may allow physicians to treat patients with DR earlier in the disease course.
ADVM-022 Gene Therapy
Developed by Adverum Biotechnologies, ADVM-022 is another intriguing gene therapy that targets the VEGF pathway. Similar to RGX-314, ADVM-022 uses an adeno-associated vector capsid, AAV.7m8; however, this gene therapy carries an aflibercept coding sequence under the control of a strong, ubiquitous expression cassette.30 With one-time intravitreal injection, ADVM-022 is designed to deliver long-term efficacy and reduce the burden of frequent anti-VEGF injections, optimize patient compliance and improve vision outcomes for patients with wet AMD and DME.
INFINITY, a Phase II trial evaluating the safety and efficacy of ADVM-022 in patients with DME, completed patient enrollment in January 2021. In INFINITY, 33 subjects will be randomized to receive a single intravitreal injection of one of the two doses of ADVM-022, or to a comparator arm of a single injection of aflibercept. The study is designed to demonstrate superior control of disease activity with ADVM-022, as shown by time to worsening of DME. All subjects will be assessed regularly and will receive additional aflibercept injections should DME disease activity progress. The primary objective is to assess the durability of a single intravitreal injection of ADVM-022. All subjects will be followed for 48 weeks after randomization. The company aims to present clinical data from the INFINITY Phase II trial in the second half of 2021.
With gene therapy, no hardware is implanted in the eye, which may circumvent potential complications such as conjunctival erosion. Moreover, there’s tremendous value in the potential role of gene therapy in preventing chronic exudative eye conditions such as DR and DME, since many researchers believe that early intervention is valuable in diabetic eye disease. While one or two intravitreal injections are generally tolerable, ongoing treatment with no definite cessation for patients who are asymptomatic can often be untenable for them. So, there’s much excitement about the possibility of a one-time treatment with sustained intraocular VEGF suppression that could slow the course of diabetic eye disease.
One potential disadvantage to gene therapy, though, is the inability to turn it off. The consequences of long-term VEGF blockade in diabetic eyes are unclear.
OPT-302
This “trap” molecule binds and neutralizes the activity of VEGF-C and VEGF-D, with the thought that combining OPT-302 with currently available anti-VEGF-A may address mechanisms of resistance associated with existing therapies.31 A Phase Ib/IIa clinical study in patients with treatment-refractory DME found a mean improvement in BCVA of 6.6 letters (n=22) from baseline to week 12 following OPT-302 + aflibercept combination treatment, compared to a gain of 3.4 letters (n=13) for patients continuing on aflibercept monotherapy.32 This visual acuity improvement accompanied a reduction in macular edema of 42.3 µm in the OPT-302 combination therapy group and 15.5 µm in the aflibercept monotherapy group. Lastly, 13.6 percent of patients in the OPT-302 combination therapy, and 7.7 percent in the aflibercept monotherapy group saw an improvement of two or more steps in their underlying diabetic retinopathy.
GB-102
GB-102 is an injectable formulation of sunitinib, a multi-targeted, receptor tyrosine kinase inhibitor that reportedly inhibits all VEGF receptor types.33 The Phase IIa trial of GB-102 in patients with DME was initiated in September 2019; it enrolled 21 patients at six clinical sites in the United States. Patients received a single intravitreal injection of either 1 or 2 mg GB-102 and are being followed for six months. The primary objective is to evaluate the safety, tolerability and pharmacodynamic response of both doses. The trial was expected to conclude in the second quarter of 2020.34
Rho Kinase Inhibitors
The Rho/Rho Kinase (ROCK) pathway promotes leukocyte adhesion to microvascular structures via increased levels of activated Intercellular Adhesion Molecule-1 (ICAM-1) and expression of other downstream proteins.35 Most clinical research has emphasized the effects of these ROCK inhibitors on lowering intraocular pressure, but a few studies have explored their potential benefits in diabetic eye disease.
Increased activity of the ROCK pathway is thought to be intertwined with the pathogenesis of DR and DME.36 One randomized clinical trial in patients with center-involving DME investigated the use of ROCK inhibitor, HA-1077 (Fasudil) from the Japanese pharmaceutical company, Asahi Kasei.37 Results demonstrated significant improvement in BCVA at three and six months in the HA-1077+bevacizumab combination group versus bevacizumab alone. Additionally, there was statistically significant improvement in central macular thickness.
Plasma Kallikrein Inhibitors
Independent from VEGF, the plasma kallikrein-kinin system pathway is activated during vascular injury; it functions by mediating factors in innate inflammation, blood flow and coagulation. This pathway offers a therapeutic target in nonresponding anti-VEGF patients. Following are some attempts to make use of this new target.
• KVD001. Patients with advanced DR were recently found to have elevated levels of components of the plasma KKS pathway.38,39 The first Phase Ib study of a plasma kallikrein inhibitor in patients with DME and suboptimal response to anti-VEGF showed that plasma kallikrein inhibition in the vitreous is generally safe and well-tolerated. Plasma kallikrein inhibitor KVD001 (Kalvista Pharmaceuticals) was intravitreally-administered in 14 patients with center-involved DME. Although not designed as an efficacy study, a trend in improved visual acuity was observed for patients receiving KVD001. However, a larger, sham-controlled, Phase II study of four monthly injections of KVD001 in 123 patients didn’t meet its primary endpoint.40
• THR-149. Another plasma KKS inhibitor in development is THR-149 by Oxurion, which functions by inhibiting the release of bradykinin in the plasma and vitreous.41-46 In September 2020, the first patient was dosed in a two-part Phase II study (KALAHARI) evaluating THR-149 for the treatment of center-involved DME. Part A (n=18) is a single-masked, dose-finding part of the study assessing three dose levels of THR-149 to select the optimal dose for Part B. Part B (n=104) is the double-masked, active-controlled part of the study with a single dose level of THR-149, and aflibercept as the comparator. Part A data is expected by mid-2021, and topline results from Part B are expected in the first half of 2023. In a previous Phase I study reported in mid-2019, THR-149 was shown to be well-tolerated and safe, with no reported dose-limiting toxicities or drug-related serious adverse events.47
Integrin inhibitors
The inhibition of integrins targets multiple processes involved in pathological angiogenesis and vascular leakage, unlike anti-VEGF treatment.
The highest-profile integrin inhibitor currently being studied is THR-687 (Oxurion), which is expected to enter Phase II testing in 2021. This pan-arginine-glycine-aspartic acid (RGD) integrin antagonist targets a broader spectrum of DR hallmarks. Preclinical models show that it is a potent inhibitor of angiogenesis-induced vascular leakage.
Oxurion has reported positive topline data from a Phase I clinical trial evaluating THR-687 for treatment of DME.48 In 2021, the company’s Phase II study of the drug will evaluate THR-687 as a VEGF-independent treatment option for treatment-naïve DME patients.
In conclusion, while anti-VEGF agents have revolutionized our treatment of both DME and DR, the field continues to evolve in the hope of providing better options for our patients. As discussed, numerous novel molecular targets may allow us to go beyond the clinical outcomes achieved by VEGF blockade, and various longer-acting pharmaceuticals might yield good results with fewer treatments, helping to improve compliance and possibly allowing us to treat more patients.
Dr. Shah is an assistant professor of ophthalmology at Tufts University School of Medicine, retina fellowship co-director at Tufts New England Medical Center/Ophthalmic Consultants of Boston, and lecturer at Harvard Medical School. He’s a sub-investigator on clinical trials with the following sponsors: NIH; Alcon; Alimera; Allergan; Genentech; Novartis; Roche; and Regeneron. Mr. Patel is a second-year medical student at UT Southwestern Medical Center.
1. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmology Retina 2018;2:1179-1187.
2. Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 2016;123:11:2376-2385.
3. ClinicalTrials.gov. Comparative effectiveness study of intravitreal aflibercept, bevacizumab, and ranibizumab for DME (Protocol T). https://clinicaltrials.gov/ct2/show/study/NCT01627249 [Accessed January 13, 2021].
4. Lim, JI. Intravitreal aflibercept injection for nonproliferative diabetic retinopathy: Year 2 results from the PANORAMA study. Invest Ophthalmol Vis Sci 2020;61:1381.
5. ClinicalTrials.gov. Study of a high-dose aflibercept in participants with diabetic eye disease (PHOTON). https://clinicaltrials.gov/ct2/show/NCT04429503 [Accessed January 13, 2021].
6. Nguyen QD, Das A, Do DV, et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 2020;127:7:963-976.
7. Drug Approval Package: BEOVU (brolucizumab-dbll). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761125_Orig1_toc.cfm [Accessed Jan 13, 2021].
8. ClinicalTrials.gov. A study of the efficacy and safety of brolucizumab vs. aflibercept in patients with visual impairment due to diabetic macular edema (KITE). https://clinicaltrials.gov/ct2/show/NCT03481660 [Accessed January 15, 2021].
9. ClinicalTrials.gov. A study of the efficacy and safety of brolucizumab vs. aflibercept in patients with visual impairment due to diabetic macular edema (KESTREL). https://clinicaltrials.gov/ct2/show/NCT03481634 [Accessed January 15, 2021].
10. ClinicalTrials.gov. Efficacy and safety of brolucizumab vs. aflibercept in patients with visual impairment due to diabetic macular edema (DME). https://clinicaltrials.gov/ct2/show/NCT03917472 [Accessed January 15, 2021].
11. ClinicalTrials.gov. Efficacy and safety of RTH258 versus aflibercept - Study 1 (HAWK). https://clinicaltrials.gov/ct2/show/NCT02307682 [Accessed January 15, 2021].
12. ClinicalTrials.gov. Efficacy and safety of RTH258 versus aflibercept - Study 2 (HARRIER). https://clinicaltrials.gov/ct2/show/NCT02434328 [Accessed January 15, 2021].
13. Khan M, Aziz AA, Shafi NA, et al. Targeting angiopoietin in retinal vascular diseases: A literature review and summary of clinical trials involving faricimab. Cells 2020;9:8:1869.
14. Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology 2019;126:8:1155-1170.
15. ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (YOSEMITE). https://clinicaltrials.gov/ct2/show/NCT03622580 [Accessed January 15, 2021].
16. ClinicalTrials.gov. A study to evaluate the efficacy and safety of faricimab (RO6867461) in participants with diabetic macular edema (RHINE). https://clinicaltrials.gov/ct2/show/NCT03622593 [Accessed January 15, 2021].
17. Roche news release. https://www.roche.com/media/releases/med-cor-2020-12-21.htm [Accessed January 15, 2021].
18. Genentech news release. https://www.gene.com/media/press-releases/14895/2021-02-11/new-phase-iii-data-show-genentechs-faricimab [Accessed March 2, 2021]
19. Campochiaro PA, Wykoff CC, Shapiro H, et al. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology 2014;121:9:1783-9.
20. Sun JK, Glassman AR, Beaulieu WT, et al. Diabetic Retinopathy Clinical Research Network. Rationale and application of the protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology 2019;126:1:87-95.
21. Roche news release. https://www.roche.com/investors/updates/inv-update-2020-05-27.htm [Accessed January 15, 2021].
22. Campochiaro PA, Marcus DM, Awh CC, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: Results from the randomized phase 2 LADDER clinical trial. Ophthalmology 2019;126:8:1141-1154.
23. ClinicalTrials.gov. Efficacy, safety, and pharmacokinetics of the port delivery system with ranibizumab in participants with diabetic macular edema compared with intravitreal ranibizumab (Pagoda). https://clinicaltrials.gov/ct2/show/NCT04108156 [Accessed January 22, 2021].
24. ClinicalTrials.gov. A multicenter, randomized study in participants with diabetic retinopathy without center-involved diabetic macular edema to evaluate the efficacy, safety, and pharmacokinetics of ranibizumab delivered via the port delivery system relative to the comparator arm (PAVILION). https://clinicaltrials.gov/ct2/show/NCT04503551 [Accessed January 22, 2021].
25. Khanani AM, Callanan D, Dreyer R, et al. End of study results for the Ladder Phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmol Retina 2020:S2468-6530:20:30447-4.
26. REGENXBIO’s gene therapy for wet amd performing encouragingly in human study. https://www.fightingblindness.org/research/regenxbio-s-gene-therapy-for-wet-amd-performing-encouragingly-in-human-study-15 [Accessed January 22, 2021].
27. Nam HJ, Lane MD, Padron E, et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol 2007;81:22:12260-12271.
28. REGENXBIO news release. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-fda-clearance-ind-phase-ii-trial-rgx-314 [Accessed January 22, 2021].
29. Initial Data Positive for Wet Age-Related Macular Degeneration Treatment. HCPLive. https://www.hcplive.com/view/initial-data-wet-age-related-macular-degeneration-treatment [Accessed January 22, 2021]
30. ClinicalTrials.gov. ADVM-022 intravitreal gene therapy for DME (INFINITY). https://www.clinicaltrials.gov/ct2/show/NCT04418427 [Accessed January 21, 2021].
31. Opthea corporate presentation. https://www.opthea.com/wp-content/uploads/2019/03/Opthea_Corporate-Presentation_2019-03.pdf [Accessed January 22, 2021]
32. Opthea news release. https://www.globenewswire.com/news-release/2020/07/27/2067722/0/en/Opthea-Reports-New-Data-of-OPT-302-in-Diabetic-Macular-Edema-at-the-2020-Annual-Meeting-of-the-American-Society-of-Retina-Specialists.html [Accessed January 22, 2021].
33. Graybug GB-102 website. https://www.graybug.vision/our-technologies-and-pipeline/#gb102 [Accessed January 22, 2021].
34. ClinicalTrials.gov. A depot formulation of sunitinib malate (GB-102) in subjects with diabetic macular edema and retinal vein occlusion. https://clinicaltrials.gov/ct2/show/NCT04085341 [Accessed January 22, 2021].
35. McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol 1995;147:3:642-53.
36. Arita R, Hata Y, Ishibashi T. ROCK as a therapeutic target of diabetic retinopathy. J Ophthalmol 2010;2010:175163
37. Ahmadieh H, Nourinia R, Hafezi-Moghadam A, et al. Intravitreal injection of a Rho-kinase inhibitor (fasudil) combined with bevacizumab versus bevacizumab monotherapy for diabetic macular oedema: A pilot randomised clinical trial. Br J Ophthalmol 2019;103:7:922-927.
38. Kita T, Clermont AC, Murugesan N, et al. Plasma kallikrein-kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes 2015;64:3588‒3599.
39. Bolinger MT, Antonetti DA. Moving past anti-VEGF: Novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016; 17:9:1498.
40. KalVista Pharmaceuticals Reports Phase 2 Clinical Trial Results in Patients with Diabetic Macular Edema. https://www.businesswire.com/news/home/20191209005372/en/KalVista-Pharmaceuticals-Reports-Phase-2-Clinical-Trial-Results-in-Patients-with-Diabetic-Macular-Edema [Accessed January 22, 2021].
41. Dugel P. THR-149 for the treatment of DME: Results of a phase I, open-label, dose-escalation study. Presented at: EURETINA; February 8, 2019; Paris.
42. Gao BB, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med 2007;13:2:181-188.
43. Clermont A, Chilcote TJ, Kita T, et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes 2011;60:5:1590-1598.
44. Lawson SR, Gabra BH, Guérin B, et al. Enhanced dermal and retinal vascular permeability in streptozotocin-induced type 1 diabetes in Wistar rats: Blockade with a selective bradykinin B1 receptor antagonist. Regul Pept 2005;124:1-3:221-224.
45. Kita T, Clermont AC, Murugesan N, et al. Plasma kallikrein-kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes 2015;64:10:3588-3599.
46. Pouliot M, Talbot S, Sénécal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One 2012;7:3:e33864.
47. Oxurion news release. https://www.globenewswire.com/news-release/2020/09/01/2086561/0/en/Oxurion-NV-Reports-First-Patient-Dosed-in-Phase-2-study-evaluating-THR-149-for-treatment-of-Diabetic-Macular-Edema-DME.html [Accessed January 22, 2021].
48. Oxurion news release. https://www.globenewswire.com/news-release/2020/02/09/1982059/0/en/Oxurion-NV-Expert-Presentation-of-Positive-Topline-Data-from-a-Phase-1-Study-evaluating-THR-687-for-the-treatment-of-DME-at-Angiogenesis-Exudation-and-Degeneration-2020-Conference.html [Accessed March 17, 2021].




