Presentation
A 14-year-old Caucasian male noted left upper eyelid swelling and droopiness that he attributed to allergies. After seven months, he presented to his local ophthalmologist due to progressive swelling. Magnetic resonance imaging of the orbits demonstrated a left lacrimal gland mass. The patient underwent incisional biopsy. Pathology revealed a pleomorphic adenoma (PA). The patient was referred to the Wills Eye Hospital Ocular Oncology Service for further management.
Medical History
Past medical history was non-contributory. Family history was notable for leukemia in the paternal grandfather, stroke in the maternal grandmother and grandfather, and hypertension in the maternal grandmother. Social history was non-contributory. The patient did not take any medications, nor did he demonstrate medication allergy.
Examination
Upon presentation to Wills’ Ocular Oncology Service, visual acuity was 20/20 in both eyes. The pupils were round, symmetric, equal and reactive to light OU. Extraocular motility and confrontational visual fields were full bilaterally. Intraocular pressure was within normal limits. Color plates were full OU.
External examination demonstrated a well-healed superior lid crease wound with 3 mm of left blepharoptosis and a palpable mass in the left superolateral orbit. There was 5 mm of painless proptosis and inferonasal displacement of the globe. Anterior examination of both eyes was within normal limits. Dilated fundoscopic examination of both eyes was unremarkable with no optic nerve edema, pallor or tortuous vessels.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
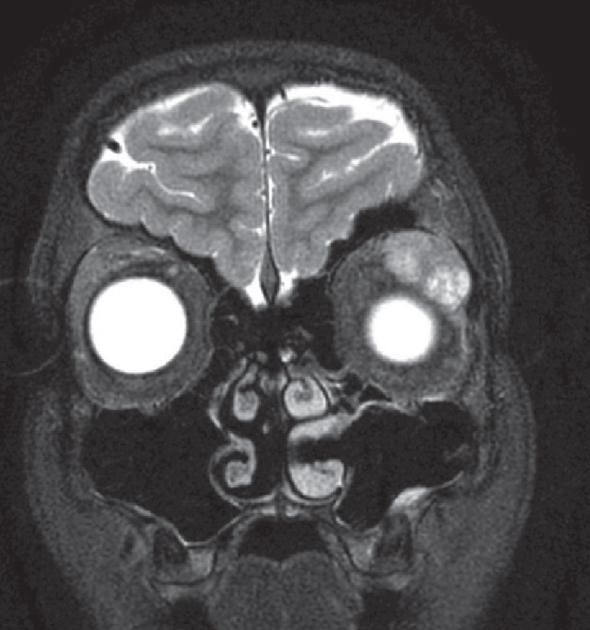 |
| Figure 1. MRI of the brain and orbits. At presentation, the T2-weighted image demonstrated a large, hyperintense mass in the left lacrimal gland. |
Review of initial orbital MRI revealed a circumscribed left lacrimal gland mass with bosselations (irregular surface) and no bony erosion (Figure 1). The differential diagnosis included lacrimal gland pleomorphic adenoma, adenoid cystic carcinoma, lymphoid hyperplasia or lymphoma, dermoid cyst, and inflammatory conditions like sarcoidosis or dacryoadenitis (inflammatory pseudotumor). Review of histopathology confirmed the diagnosis of pleomorphic adenoma. Subsequently, complete surgical excision of the mass via lateral orbitotomy approach was recommended.
Surgical approach revealed the large lacrimal gland mass with orbital scarring from the previous incisional biopsy. The mass was completely excised. Histopathology confirmed pleomorphic adenoma with no evidence of malignancy.
Periodic follow-up MRIs were performed to monitor the site, given the previous incisional biopsy. At 29 months follow up, increasing ptosis was noted. Repeat MRI revealed concern for recurrent left lacrimal gland tumor. Orbitotomy and pathology revealed recurrent pleomorphic adenoma with no evidence of malignancy. Given the recurrence, stereotactic radiotherapy (SRT) (25Gy) was performed to the superior and lateral orbit. Repeat MRI 2.5 years after SRT demonstrated a second recurrence in the superior medial orbit. Surgical resection and histopathology demonstrated multifocal recurrent pleomorphic adenoma, but with transformation to cellular atypia, composed of variably cellular tumor nodules (Figure 2). The patient received SRT (25 Gy) to the medial half of the orbit. Six months later, MRI scan again noted a recurrent mass in the inferior temporal orbit, which was surgically removed, and histopathology demonstrated multifocal pleomorphic adenoma with foci of transformation to carcinoma ex pleomorphic adenoma (CXPA) (Figure 3). After discussion of management options with the patient and at a multidisciplinary tumor board, a recommendation for orbital exenteration was suggested and performed.
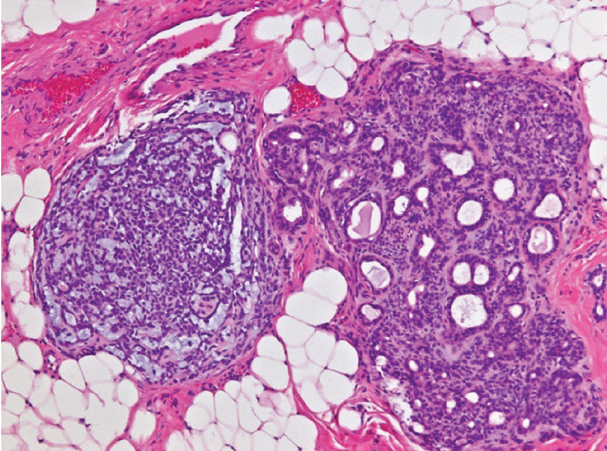 |
| Figure 2. Pleomorphic adenoma with atypia. The resected tumor revealed a neoplasm composed of two morphologically distinct nodules. The nodule on the right demonstrates classic features of PA, comprising bilayered ductules in an abundant stroma with scattered myoepithelial cells. The nodule on the left is composed of cellular myoepithelial cell proliferation. (Hematoxylin-eosin; original magnification x200.) |
Discussion
Lacrimal fossa lesions represent more than 10 percent of all orbital space-occupying lesions.1 Despite its overall rare prevalence, pleomorphic adenoma, also termed benign mixed tumor, is the most common benign epithelial neoplasm of the lacrimal gland.2,3 Pleomorphic adenoma often presents in the fourth decade with no appreciable—or possibly slight male—sex predilection.3,4 Patients present classically with longstanding painless proptosis and inferonasal displacement of the globe.5 Imaging demonstrates a well-circumscribed mass in the lacrimal gland fossa, which may be associated with bone remodeling, without bony erosion or destruction.
Histopathologically, pleomorphic adenoma is pseudoencapsulated and consists of bilayered epithelial-myoepithelial ductules in the background of myoepithelial cell proliferation and variably myxoid and cartilaginous stroma.6 Foci of squamous differentiation can be seen. Dense cellularity, cytologic atypia, mitotic figures, and necrosis are not features of benign pleomorphic adenoma. Incompletely excised tumors tend to recur in a multifocal, multinodular fashion, no longer bounded by a pseudocapsule. Pleomorphic adenomas commonly demonstrate overexpression of the pleomorphic adenoma gene 1 (PLAG1) or high-mobility group AT-hook 2 (HMGA2) genes, which correspond to underlying PLAG1 and HMGA2 gene rearrangements.7 Immunohistochemically, PLAG1 nuclear staining is highly specific for pleomorphic adenoma.6
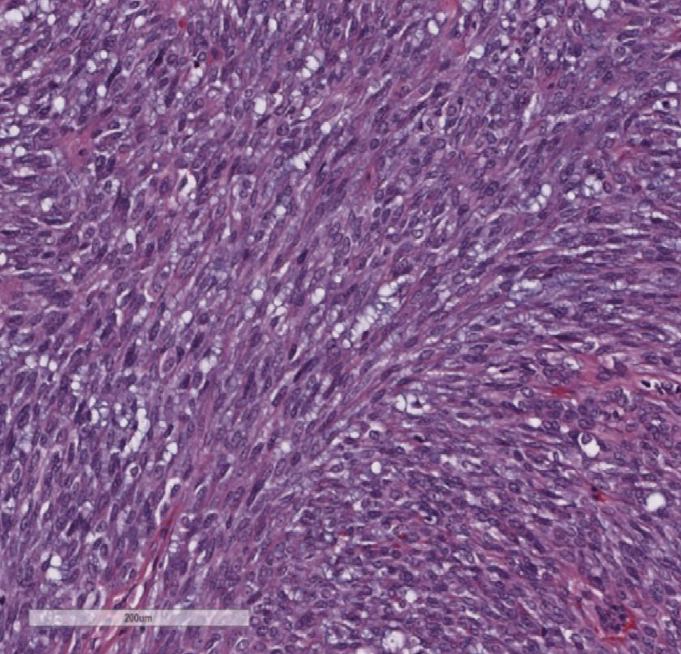 |
| Figure 3. Carcinoma ex pleomorphic adenoma (CXPA). The resected tumor revealed markedly cellular proliferation of pleomorphic spindle cells with hyperchromatic overlapping nuclei, compatible with myoepithelial CXPA. |
In most cases, pleomorphic adenoma demonstrates an indolent course and is cured with complete excision. However, malignant transformation (CXPA, or malignant mixed tumor) has been reported in 1.5 to 13.8 percent of pleomorphic adenomas.8,9 Malignancy can arise spontaneously or in a setting of recurrence following incomplete excision.10,11 Although some experts contest a strict “no incisional biopsy” dogma, consensus remains in favor of complete primary excision of the tumor with intact pseudocapsule without biopsy.11-14 According to some studies, CXPAs are more frequent in males.15 Patients typically present with proptosis, orbital ache, palpable mass and reduced ocular motility.16 Imaging can demonstrate an infiltrative mass with bony erosion.16
At a microscopic level, CXPAs can show patchy to complete hyalinization (“mummification”), invasion into the surrounding myxoid stroma, and necrosis.16 The carcinoma component can be of any type in CXPA. In lacrimal CXPA, lacrimal duct adenocarcinoma is the most common CXPA. Myoepithelial carcinoma XPA is relatively rare. These tumors can demonstrate underlying molecular genetic rearrangements in PLAG1 and HMGA2 genes, similar to their precursor pleomorphic adenoma, which may aid in the diagnosis in challenging cases.16,17 Prognosis is based on the following criteria: 1) in situ carcinoma (intracapsular); 2) minimally invasive (<1.5 mm); and 3) invasive (≥1.5 mm).17 Treatment is ultimately rendered based on histopathologic findings and disease staging, and largely consists of surgical excision, exenteration or radiation therapy.16-18 One retrospective study showed that patients who underwent complete excision with pseudocapsule intact have greater survival as compared to those who underwent debulking and radiation therapy.16
Our case highlights the importance of initial complete surgical resection of pleomorphic adenoma. Incomplete excision is a risk for multifocal recurrence, which is extremely difficult to manage and poses a risk for malignant transformation. Long-term follow up of patients who have undergone resection of pleomorphic adenoma with clinical examination and MRI is prudent. New onset blepharoptosis, proptosis, pain or dysmotility or MRI findings of a recurrent mass should raise consideration for CXPA.
1. Shields JA, Bakewell B, Augsburger JJ, Flanagan JC. Classification and incidence of space-occupying lesions of the orbit. A survey of 645 biopsies. Arch Ophthalmol 1984;102:11:1606-11.
2. Shields CL, Shields JA, Eagle RC, Rathmell JP. Clinicopathologic review of 142 cases of lacrimal gland lesions. Ophthalmology 1989;96:4:431-5.
3. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology 2004;111:5:997-1008.
4. Liu R, Wang N, Zhang H, Ge X, Ma JM. Clinical analysis of 109 cases of lacrimal gland pleomorphic adenoma. Int J Ophthalmol 2021;18:14:12:1852-1857.
5. Bernardini FP, Devoto MH, Croxatto JO. Epithelial tumors of the lacrimal gland: An update. Curr Opin Ophthalmol 2008;19:5:409-13.
6. Mendoza PR, Jakobiec FA, Krane JF. Immunohistochemical features of lacrimal gland epithelial tumors. Am J Ophthalmol 2013;156:6:1147-1158.
7. Andreasen S, von Holstein SL, Homøe P, Heegaard S. Recurrent rearrangements of the PLAG1 and HMGA2 genes in lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Acta Ophthalmol 2018;96:7:e768-e771.
8. Antony J, Gopalan V, Smith RA, Lam AK. Carcinoma ex pleomorphic adenoma: A comprehensive review of clinical, pathological and molecular data. Head Neck Pathol 2012;6:1:1-9.
9. Zbären P, Zbären S, Caversaccio MD, Stauffer E. Carcinoma ex pleomorphic adenoma: Diagnostic difficulty and outcome. Otolaryngol Head Neck Surg 2008;138:5:601-5.
10. Shields JA, Shields CL. Malignant transformation of presumed pleomorphic adenoma of lacrimal gland after 60 years. Arch Ophthalmol 1987;105:10:1403-5.
11. Currie ZI, Rose GE. Long-term risk of recurrence after intact excision of pleomorphic adenomas of the lacrimal gland. Arch Ophthalmol 2007;125:12:1643-6.
12. Lai T, Prabhakaran VC, Malhotra R, Selva D. Pleomorphic adenoma of the lacrimal gland: Is there a role for biopsy? Eye (Lond) 2009;23:1:2-6.
13. Rose GE, Wright JE. Pleomorphic adenoma of the lacrimal gland. Br J Ophthalmol 1992;76:7:395-400.
14. Auran J, Jakobiec FA, Krebs W. Benign mixed tumor of the palpebral lobe of the lacrimal gland. Clinical diagnosis and appropriate surgical management. Ophthalmology 1988;95:1:90-9.
15. Wang XN, Qian J, Yuan YF, Zhang R, Zhang YQ. Space-occupying lesions of the lacrimal gland at one tertiary eye center in China: A retrospective clinical study of 95 patients. Int J Ophthalmol 2012;5:2:208-11.
16. Vahdani K, Rose GE. Carcinoma expleomorphic adenoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg 2021;37:6:576-582.
17. Tom A, Bell D, Ford JR, Debnam JM, Guo Y, Frank SJ, Esmaeli B. Malignant mixed tumor (carcinoma ex pleomorphic adenoma) of the lacrimal gland. Ophthalmic Plast Reconstr Surg 2020;36:5:497-502.
18. Woo KI, Yeom A, Esmaeli B. Management of Lacrimal Gland Carcinoma: Lessons From the Literature in the Past 40 Years. Ophthalmic Plast Reconstr Surg 2016;32:1:1-10.



