|
|
Artificial intelligence is expected to provide insight into the causes of dry eye as well as aid diagnostics and treatment development. Experts say future clinical decision-making tools will help meet the care demands of this growing population. |
Dry eye is a difficult disease to pin down, considering its many possible etiologies, signs and symptoms. Artificial intelligence promises to aid dry-eye diagnosis and treatment, but it too faces several hurdles to “understanding” this condition, from interpreting complex imaging1 to training without a set of unified diagnostic criteria, which also limits the ability to compare performance among studies.
Despite these challenges, AI research in dry eye is expected to grow in the following areas: improvement of proposed grading by dry-eye platforms; objective quantification of qualitative measurements; and the establishment of severity or prognosis scores through multi-modal approaches.2
Adding more objectivity to qualitative testing will not only improve the power of dry-eye studies but will also enable more patients to receive accurate and efficient diagnoses by more clinicians. “There are the standard clinical tests for dry eye, but these tests are subjective and making the diagnosis requires a certain level of interpretation expertise,” says Stephen Pflugfelder, MD, a professor and the James and Margaret Elkins Chair in Ophthalmology at Baylor College of Medicine in Houston. “Artificial intelligence could help in this regard. While it isn’t [widely] used in the dry-eye clinic space yet, it may be in the future to aid diagnosis and provide clinical decision support. It’s an exciting area.”
Here, we’ll review some of the latest areas of AI research in dry-eye disease.
Analyzing the Tear Film
Loss of tear-film homeostasis, ocular surface inflammation, hyperosmolarity and quality-of-life issues such as eye irritation and visual disturbances are hallmarks of dry-eye disease,3 but as clinicians know, the signs of dry eye don’t always correlate with the symptoms described by patients. No single clinical test can definitively diagnose dry-eye disease.3 Instead, multiple subjective tests, such as Schirmer’s, tear breakup time and dye staining with fluorescein and lissamine green, are employed along with patient-reported symptoms and use of questionnaires. As experts point out, processing all of this data from multiple modalities takes considerable time and skill, but AI could help. Here’s how the algorithms are evolving:
• Pooling clinical tests. Machine learning classification algorithms for detecting tear-film breakup time showed good accuracy in a small pilot study published last year in Nature.4 Researchers tested multiple algorithms on retrospectively collected data of 431 patients from a Norwegian dry-eye clinic, which included ocular surface staining, meibomian gland expressibility and dropout, meibum quality, meiboscore, blink frequency and tear osmolarity. The algorithms identified age, ocular surface staining, Schirmer’s test and OSDI as the most important predictors associated with tear-film instability, followed by meibomian gland characteristics.
• Slit lamp cameras. This year, researchers developed an algorithm to estimate tear-film breakup time from slit lamp videos recorded using a portable device of their own invention (Smart Eye Camera).5 The algorithm was trained on 16,440 fluorescence-enhanced blue light ocular frames annotated for tear-film breakup by a dry-eye specialist. A case was diagnosed as having dry-eye disease when the model estimated tear-film breakup time ≤5 seconds and OSDI input >13. Using the Asia Dry Eye Society diagnostic criteria for 22,172 annotated frames (158 eyes of 79 patients), the algorithm demonstrated tear-film breakup estimation accuracy of 0.789 and an area under the curve of 0.877. Sensitivity and specificity were 0.778 and 0.857, respectively.
 |
|
Check out last year’s issue of Review of Cornea & External Disease for an in-depth exploration of artificial intelligence for the cornea, available here. |
• Anterior segment OCT. Several AI models have been developed to detect dry-eye disease from tear meniscus parameters using anterior segment OCT images. A 2021 paper describing a deep-learning model for AS-OCT and dry eye reported reliable autonomous diagnosis capabilities.6 The model was trained and tested on 27,180 AS-OCT images collected prospectively from 151 eyes of 91 patients. Clinical dry-eye tests were performed in the DED group for comparison. The model achieved an accuracy of 84.62 percent, sensitivity of 86.36 percent and specificity of 82.35 percent for DED diagnosis. The mean DED probability score was 0.81 ±0.23 in the DED group and 0.20 ±0.27 in the control group (p<0.01). Diagnosis accuracy was significantly better with the model than with corneal staining, conjunctival staining and Schirmer’s test (<0.05). There were significant differences between the model’s diagnostic accuracy compared with OSDI and TBUT.
Other deep-learning models for AS-OCT-based diagnosis include a model trained on 158,220 images from 879 eyes of 478 participants that had an AUC > 0.99, an area under precision-recall curve >0.96 and F1 scores >0.90 for DED diagnosis;7 a random forest regression-based multivariable diagnosis model with corneal epithelial mapping data that had high sensitivity (86.4 percent) and specificity (91.7 percent), suggesting that adding corneal epithelial mapping data may improve DED diagnostic accuracy;8 and a deep-learning model for segmenting the lower tear meniscus.9
The lower tear meniscus segmentation model trained deep convolutional neural networks on 6,658 images labeled by a thresholding-based segmentation algorithm.9 Two approaches were compared: one that directly segmented the tear meniscus and another that first localized the region of interest and then performed segmentation at a higher resolution of an image section. The five-fold cross-validation showed a sensitivity of 96.36 percent and 96.43 percent, for each approach, respectively; a specificity of 99.98 percent and 99.86 percent; and a Jaccard index of 93.24 percent and 93.16 percent.
• Interferometry. Few studies using interferometry have been published to date, but experts say AI analysis of the tear film has potential as a screening tool once technological improvements in image processing, such as better resolution and contrast, make AI analysis easier.10
Researchers in Japan created an AI-based diagnosis model for the DR-1α tear interferometer (Kowa), which was previously shown to subtype dry eye based on fringe patterns as normal tear condition, aqueous-deficient dry eye or evaporative dry eye.11 Their model was constructed using 11 predetermined image features to distinguish the three subtypes, obtained using the same instrument, and trained on 138 images of each type as well as control images. The model was tested on 100 interferometric movies obtained from controls or dry-eye patients. The group reported reliable AI diagnosis, with F-scores of 0.954, 0.806 and 0.762 for aqueous-deficient, evaporative and normal pattern types.
In 2020, a group in Brazil proposed a computational method to address the challenges related to classifying lipid layer interference patterns.12 In the automated system, the region of interest is segmented, and features are extracted using phylogenetic diversity indexes. Interferometry images are then classified using Support Vector Machines, Random Forest, Naive Bayes, Multilayer Perceptron, Random Tree and RBFNetwork. Finally, results are validated. The method demonstrated 97.54-percent accuracy, an AUC of 0.99, a Kappa index of 0.96 and F-score of 0.97.
• Keratography. A deep transfer learning model was able to directly identify dry-eye disease using ocular surface video frames with an area under the curve of 0.98.13 The 2023 retrospective study included 244 ocular surface videos (Keratograph 5M, Oculus) of 244 eyes (116 normal eyes; 128 with dry-eye disease) to assess the tear film. According to the study, network activation maps showed that the lower paracentral cornea was the most important region for detecting dry eye in the CNN model.
Blink Pattern Analysis
Blinking is a multifaceted process affected by numerous factors, from psychological and emotional states to fatigue,14 mental activity and age.15 Studies report that individuals with dry-eye disease have altered blinking patterns from those without the condition.15
AI is expected to elucidate the complex patterns of blinks in dry-eye patients by aiding the measurement and assessment of blink parameters, which are currently challenging to analyze due to rapid blink speed (<100 miliseconds) and the continuous changes and phases of the blink process.15
Researchers in Beijing used a machine learning model to record spontaneous blink patterns. The model, built using UNet image segmentation and ResNet image classification algorithms, showed that dry-eye patients had more partial blinks, fewer complete blinks and a shorter duration of the eyelid closure phase compared with controls.16
A total of 357 dry-eye patients and 152 controls were included. Participants completed the following tests: OSDI questionnaire; blink video capture; lipid layer thickness; tear break-up time; fluorescein staining; and Schirmer II test.
The models analyzed single frames of the blink videos and used the palpebral opening height of each frame to establish a spontaneous blink wave. The segmentation and classification models each had an accuracy of 96 percent. Consistency with manual analysis was 97.9 percent.
The researchers reported that the average number of blinks for dry-eye patients was 30 per minute compared with controls’ 20 per minute (p=0.002). Complete blinks for dry-eye patients averaged six per minute vs. 12 per minute (p=0.016); partial blinks for dry-eye patients averaged 15 per minute vs. three per minute (p<0.001). No significant differences were found in average interblink interval or eyelid opening phases, but dry-eye patients had a significantly shorter eyelid-closed phase than controls (0.8 seconds vs. 1.3 seconds, p=0.006).
According to a deep learning model for analyzing blink videos (using the Keratograph 5M), a frame rate of ≥30 frames per second is optimal.17 The case-controlled study included 50 dry-eye disease patients and 50 controls. Participants filled out symptom questionnaires and also underwent ocular surface assessments. The model processed videos and create blink profiles to enable comparison of blink parameters and association with dry-eye signs and symptoms. The researchers reported that blink parameters based on 30-fps videos had higher sensitivity and accuracy than videos based on 8 fps.
Additionally, the model showed that average relative interpalpebral height and the frequency and proportion of incomplete blinking were significantly higher among dry-eye participants than controls (p<0.001). Incomplete blinking, proportion of incomplete blinks and average interpalpebral height were also associated with dry-eye signs and symptoms.
Meibomian Gland Assessment
Deep learning models are performing meibomian gland segmentation, a necessary initial step for further automated image-based analysis; speeding up meibography image evaluation; and learning to differentiate between types of meibomian gland dysfunction to aid diagnosis.
• Meibography. A deep-learning approach developed in 2019 by researchers in Berkley, California, automatically segments the total eyelid and meibomian gland atrophy regions to provide quantitative information on gland atrophy from meibography images.18 The researchers collected 706 meibography images and corresponding meiboscores. Images were annotated with eyelid and atrophy regions and used to train (n=497 images) and evaluate (n=209 images) the model.
The algorithm achieved a mean 95.6-percent meiboscore grading accuracy, which outperformed the lead clinical investigator by 16 percent and the clinical team by 40.6 percent. For eyelid and atrophy segments, the algorithm achieved 97.6-percent and 95.4-percent accuracies, respectively; and 95.5-percent and 66.7-percent mean intersection over union accuracies, respectively. The model’s average root-mean-square deviation of the percent atrophy prediction was 6.7 percent.
In 2022, a deep-learning method for segmenting meibomian glands and eyelids demonstrated the ability to automatically detect all individual meibomian glands and quantify the meibomian gland area and area ratio.19 The South Korean study included 1,600 meibography images taken in a clinical setting, 1,000 of which were annotated with multiple revisions by investigators and then graded six times by MGD experts. The group trained two deep-learning models separately to segment areas of the meibomian glands and eyelid in order to estimate meiboscores and meibomian gland ratio. They employed a generative adversarial network to remove specular reflections from the raw images without affecting grading.
The model demonstrated mean ratio of meibomian gland calculations consistent with those of the investigator—26.23 vs. 25.12 percent in the upper eyelids and 32.34 vs. 32.29 percent in the lower eyelids, respectively. Model accuracy was 73.01 percent for meiboscore classification on the validation set. It achieved 59.17-percent accuracy on images from an independent center compared with MGD experts’ 53.44-percent accuracy.
Using a Mask R-convolutional neural network deep learning framework, researchers developed a meibography image grading aid to help save specialists’ time.20 The model was established using 1,878 manually annotated meibography images (in two classes: conjunctiva and meibomian glands), and an independent test dataset of 58 images was used to compare accuracy and efficiency against specialists. Performance was evaluated by validation loss (loss value of the verification dataset, where a smaller value indicates a better training result) and mean average precision ([mAP] the mean value of average precisions for each class, demonstrating the accuracy of area detection and segmentation on the validation dataset).
The model predicted meibomian gland loss ratio with high accuracy in the conjunctiva (validation loss <0.35; mAP >0.976) and in meibomian glands (validation loss <1, mAP >0.92). The difference between specialist evaluation and the AI model was minimal. The model evaluated images in 480 milliseconds—21x faster than a human.
• In-vivo confocal laser microscopy. A deep-learning model developed in Japan successfully differentiated between healthy meibomian glands and obstructive MGD using in-vivo confocal laser microscopy images.21 Nine different network structures and single and ensemble deep-learning models were constructed and trained using 137 images from 137 individuals with obstructive MGD and 84 images from 84 controls. The single deep-learning model (DenseNet-201)
diagnosed obstructive MGD with an AUC of 0.966, a sensitivity of 94.2 percent and a specificity of 82.1 percent. The ensemble deep-learning model (VGG16, DenseNet-169, DenseNet-201 and InceptionV3) had an AUC of 0.981, a sensitivity of 92.1 percent and a specificity of 98.8 percent.
In a larger study from China, a convolutional neural network trained on in-vivo confocal laser microscopy images differentiated obstructive MGD, atrophic MGD and normal meibomian glands.22 The model, trained on 4,985 images and validated on 1,663 images, was tested by comparing its image-based identification of meibomian glands to diagnoses made by an expert. The study included 2,766 healthy controls, 2,744 participants with obstructive MGD and 2,801 participants with atrophic MGD. Differential diagnostic accuracy was highest using DenseNet169 model (>97 percent). The model had sensitivities and specificities of 88.8 percent and 95.4 percent, respectively, for obstructive MGD and 89.4 percent and 98.4 percent, respectively, for atrophic MGD.
Early Clinical Innovations
 |
|
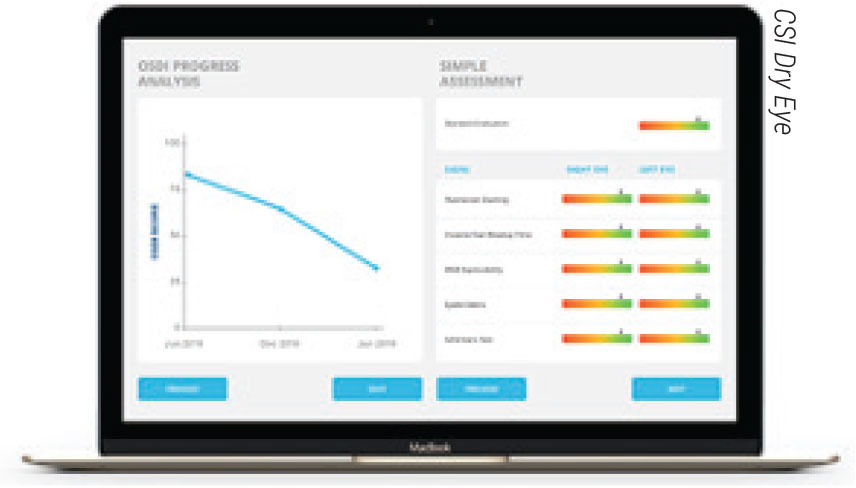
CSI Dry Eye is a cloud-based artificial intelligence platform for clinicians that assesses clinical test results to formulate diagnoses and recommend treatments. The company says the software can increase practice efficiency and boost diagnostic accuracy. |
Dry-eye AI tools aren’t yet ready for widespread clinical adoption, but that doesn’t mean they haven’t been incorporated at all. Here are some ways AI is beginning to enter the dry-eye clinic space:
• University partnerships. Some private practices are working with larger research institutions to develop AI-based tools for their clinics. Belfast-based Cathedral Eye Clinic has partnered with Aston University in Birmingham, England, to develop an AI-based digital decision support system for the ocular surface. The AI tool will analyze patient clinical data and aid clinicians in diagnosing eye diseases and developing treatment strategies. According to Aston University, key aspects of the program include exploring the impact of ocular surface issues on refractive outcomes after laser- and lens-based treatments in addition to identifying preoperative clinical management techniques to improve outcomes.23
• A cloud-based platform. CSI Dry Eye is a newcomer to the clinic space that focuses on dry-eye diagnostics and treatment, using support vector machine learning technology to create dry-eye type and severity models. The platform proposes a diagnosis based on clinical test result input and multiple patient questionnaires. The company says the platform saves time, boosts patient retention, and increases practice productivity and diagnostic accuracy. To learn more, visit csidryeye.com.
The Takeaway
AI is slated to offer more objective and consistent diagnoses and disease severity stratification,24 as well as provide insight into etiologies and the complex relationships between the many factors that contribute to dry eye.25 The efficiency promised by such automation is also expected to help ameliorate the high economic26 and quality-of-life27 burden associated with DED.
1. Zhang Z, Wang Y, Zhang H, et al. Artificial intelligence-assisted diagnosis of ocular surface diseases. Front Cell Dev Biol 2023;11:1133680.
2. Brahim I, Lamard M, Benyoussef A and Quellec G. Automation of dry eye disease quantitative assessment: A review. Clin Exp Ophthalmol 2022;50:653-666.
3. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. The Ocular Surface 2017;15:3:334–365.
4. Fineide F, Storås AM, Chen X, et al. Predicting an unstable tear film through artificial intelligence. Scientific Reports 2022;12:21416.
5. Shimizu E, Ishikawa T, Tanji M, et al. Artificial intelligence to estimate the tear film breakup time and diagnose dry eye disease. Sci Rep 2023;13:1:5822.
6. Chase C, Elsawy A, Eleiwa T, Ozcan E, Tolba M, Shousha MA. Comparison of autonomous AS-OCT deep learning algorithm and clinical dry eye tests in diagnosis of dry eye disease. Ophthamology 2021;15:4281-4289.
7. Elsawy A, Eleiwa T, Chase C, et al. Multidisease deep learning neural network for the diagnosis of corneal diseases. Am J Ophthalmol 2021;226:252-261.
8. Edorh NA, El Maftouhi A, Djerada Z, et al. New model to better diagnose dry eye disease integrating OCT corneal epithelial mapping. British Journal of Ophthalmology 2022;106:1488-1495.
9. Stegmann H, Werkmeister RM, Pfister M, et al. Deep learning segmentation for optical coherence tomography measurements of the lower tear meniscus. Biomed Optics Express 2020;11:3:1539-1554.
10. Yang H, Che S, Hyon J and Han S. Integration of artificial intelligence into the approach for diagnosis and monitoring of dry eye disease. Diagnostics 2022;12:3167.
11. Arita R, Yabusaki K, Yamauchi T, et al. Diagnosis of dry eye subtype by artificial intelligence software based on the interferometric fringe pattern of the tear film obtained with the Kowa DR-1α instrument. Invest Ophthalmol Vis Sci 2018;59:1965.
12. da Cruz LB, Carvalho Souza J, Alves de Sousa J, et al. Interferometer eye image classification for dry eye categorization using phylogenetic diversity indexes for texture analysis. Computer Methods and Programs in Biomedicine 2020;188:105269.
13. Abdelmotaal H, Hazarbasanov R, Taneri S, et al. Detecting dry eye from ocular surface videos based on deep learning. Ocul Surf 2023;28:90-98.
14. Rodriguez JD, Lane KJ, Ousler GW, Angjeli E, Smith LM, Abelson MB. Blink: Characteristics, controls, and relation to dry eyes. Curr Eye Res 2018;43:1:52-66.
15. Su Y, Liang Q, Su G, et al. Spontaneous eye blink patterns in dry eye: clinical correlations. Invest Ophthalmol Vis Sci 2018;59:12:5149-5156.
16. Zhang ZZ, Kuang RF, Wei ZY, et al. [Detection of the spontaneous blinking pattern of dry eye patients using the machine learning method]. Zhonghua Yan Ke Za Zhi [Chinese J Ophthalmol] 2022;58:2:120-129.
17. Zheng Q, Wang L, Wen H, et al. Impact of incomplete blinking analyzed using a deep learning model with the keratograph 5m in dry eye disease. Transl Vis Sci Technol 2022;11:3:38.
18. Wang J, Yeh TN, Chakraborty R, et al. A deep learning approach for meibomian gland atrophy evaluation in meibography images. Trans Vis Sci Tech. 2019;8:6:37.
19. Saha RK, Chowdhury AM, Na K, eta l. Automated quantification of meibomian gland dropout in infrared meibography using deep learning. The Ocular Surface 2022;26:283-294.
20. Yu Y, Zhou Y, Tian M, et al. Automatic identification of meibomian gland dysfunction with meibography images using deep learning. Int Ophthalmol 2022;42:11:3275-3284.
21. Maruoka S, Tabuchi H, Nagasato D, et al. Deep neural network-based method for detecting obstructive meibomian gland dysfunction with in vivo laser confocal microscopy. Cornea 2020;39:6:720-725.
22. Zhang Y, Zhao H, Lin J, et al. Artificial intelligence to detect meibomian gland dysfunction from in-vivo laser confocal microscopy. Front Med 2021;8:e774344.
23. Aston University. Knowledge Transfer Partnerships at work. https://www.aston.ac.uk/business/collaborate-with-us/knowledge-transfer-partnership/at-work/cathedral-eye-clinic. Accessed April 20, 2023.
24. Storås AM, Strümke I, Riegler MA, et al. Artificial intelligence in dry eye disease. Ocular Surface 2022;23:74-86.
25. Ji Y, Liu S, Hong X, et al. Advances in artificial intelligence applications for ocular surface diseases diagnosis. Frontiers in Cell and Developmental Biology 2022;10.1107689.
26. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 2011;30:4:379-387.
27. Morthen MK, Magno MS, Utheim TP, Snieder H, Hammond CJ, Vehof J. The physical and mental burden of dry eye disease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul Surf 2021;21:107-117.