Peter N. Youssef, MD
Michael S. Ip, MD
With the increasing use of pharmacotherapy to treat ophthalmic disease, the application of such strategies has been applied to the treatment of branch and central retinal vein occlusions. Recent randomized, prospective trials have examined the role of agents such as corticosteroids and anti-vascular endothelial growth factor versus more traditional forms of therapy such as macular grid photocoagulation and observation. These trials have provided many answers regarding the use of pharmacotherapy for the treatment of these disorders and have allowed vitreoretinal specialists to employ an evidence-based approach in treating patients with branch and central retinal vein occlusions.
BVOS & CVOS
Until recently, the treatment of branch retinal vein occlusion and central retinal vein occlusion has been guided primarily by the results of the Branch Vein Occlusion Study (BVOS) and the Central Vein Occlusion Study (CVOS). The BVOS and CVOS, both randomized, prospective, controlled studies, were designed in part to determine whether the use of laser photocoagulation is beneficial in treating macular edema secondary to retinal vein occlusion.
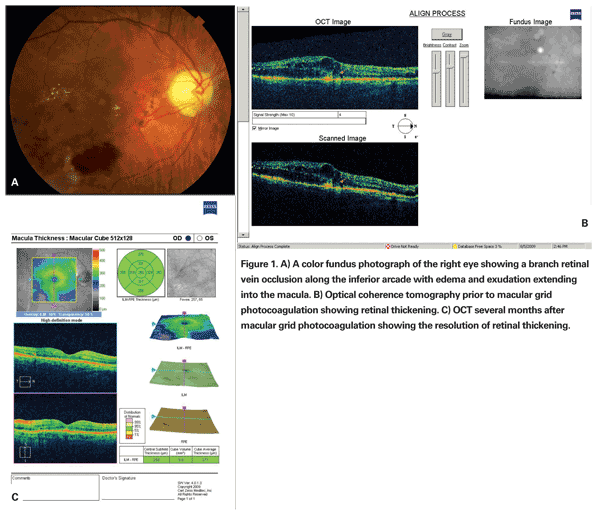
The BVOS trial established that in a subgroup of patients with intact foveal vascularity and visual acuity ranging from 20/40 to 20/200, argon laser photocoagulation applied as macular grid photocoagulation improved visual acuity compared with those in the control group not receiving treatment. Treated eyes were more likely to gain two lines of visual acuity (65 percent) compared with eyes in the untreated control group (37 percent). Additionally, treated eyes were more likely to attain 20/40 or better vision at the three years' follow-up, with a mean visual acuity improvement of 1.3 ETDRS lines in treated eyes versus 0.2 ETDRS lines in eyes in the observational control group. The results of the BVOS established macular grid photocoagulation as the standard therapy for the treatment of branch retinal vein occlusions with persistent macular edema and decreased visual acuity (See Figure 1).1
The CVOS trial examined the potential role for macular grid photocoagulation in central retinal vein occlusion. The CVOS was able to define and stratify patients into ischemic and nonischemic occlusions based on the extent of capillary nonperfusion on fluorescein angiography and demonstrate better overall visual outcomes in the nonischemic CRVO group. However, in contrast to the findings in the BVOS, macular grid photocoagulation did not confer a statistical improvement in visual acuity versus the observation group in the CVOS trial.2
Corticosteroid vs. Laser
Reports of the use of periocular and intravitreal corticosteroids to attain improvement in visual acuity and a reduction in macular edema secondary to branch and central vein occlusions spurred interest in the use of these medications for the treatment of vein occlusions.3-7 Corticosteroids have been shown to reduce both inflammation and the production of VEGF, which are thought to play a role in the increased retinal capillary permeability and consequent macular edema. While many anecdotal and retrospective studies seemed to support the use of triamcinolone for the treatment of vein occlusions, randomized prospective data was unavailable until the completion of The Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study. The SCORE study was a randomized, prospective, multicenter trial designed to compare the outcomes of intravitreal triamcinolone injection to standard of care laser photocoagulation and observation for the treatment of macular edema secondary to branch retinal vein occlusion and central retinal vein occlusion respectively.
The SCORE study compared the use of 1-mg and 4-mg doses of intravitreal triamcinolone acetonide to macular grid photocoagulation for the treatment of macular edema secondary to branch retinal vein occlusion. The primary outcome measured was a gain in visual acuity of 15 or more letters from baseline to month 12. While 28.9 percent of the patients in the standard of care, 25.6 percent in the 1-mg intravitreal triamcinolone, and 27.2 percent in the 4-mg intravitreal triamcinolone met the primary outcome measure respectively, there was no statistically significant difference between any of the treatment arms. Additionally, the SCORE study examined the safety profiles between the 1-mg and 4-mg doses of intravitreal triamcinolone acetonide and grid photocoagulation.
Due to the relatively higher percentage of patients requiring intraocular pressure-lowering meds, increased rate of cataract formation, post-injection deposition of intravitreal silicone oil droplets and possibility of endophthalmitis in the 1-mg and 4-mg intravitreal triamcinolone groups, macular grid photocoagulation was judged to have a superior safety profile when compared to the treatment with the injection of intravitreal triamcinolone. The authors of the SCORE-BRVO trial therefore recommend that macular grid photocoagulation remain the treatment of choice for reduced visual acuity secondary to macular edema in the cases of branch retinal vein occlusion.8
The SCORE-CRVO trial compared the use of intravitreal injections of 1 mg and 4 mg of triamcinolone versus observation in the treatment of central retinal vein occlusion. The data from the SCORE-CRVO trial demonstrated the superiority of intravitreal triamcinolone with respect to visual acuity improvement when compared to observation with 7 percent of patients in the observation group, 27 percent in the 1-mg triamcinolone group, and 26 percent in the 4-mg triamcinolone group gaining 15 or more letters from baseline to 12 months. As with the BRVO data, patients treated with intravitreal triamcinolone were more likely to demonstrate progression of cataracts and the need for IOP-lowering medication when compared to the observation group. Because there was no statistically significant difference in the primary outcome measure when comparing the 1-mg and 4-mg triamcinolone groups, and the 1-mg group had a superior safety profile when compared to the 4-mg group, the authors recommended the use of 1-mg triamcinolone intravitreal injection for the treatment of non-ischemic central retinal vein occlusion.9
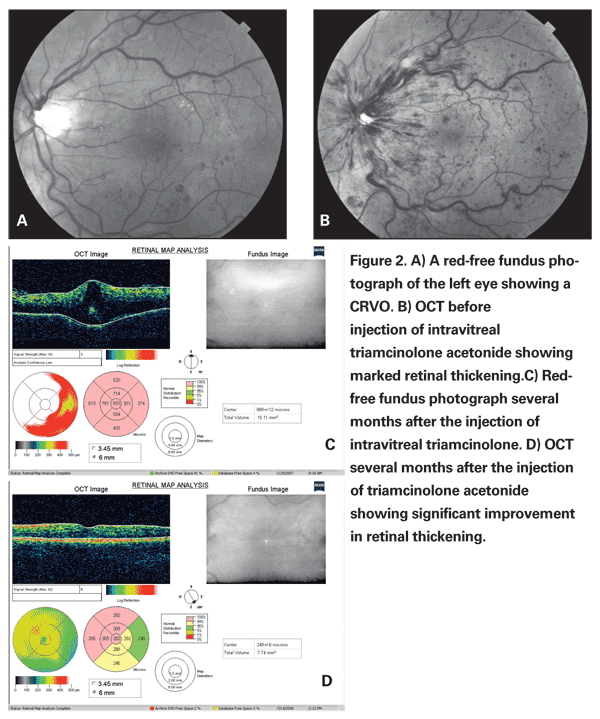
The SCORE Study provided useful information regarding the use of intravitreal triamcinolone in the treatment of branch and central retinal vein occlusion. As evidence from the SCORE Study suggests, treatment of CRVO often required a series of injections over time. This raises the possibility of the potential advantage of sustained-release delivery devices for the administration of intraocular corticosteroids. As these devices and techniques become more readily available, the use of corticosteroids may gain wider usage in the treatment of these conditions.10
Anti-VEGF vs. Laser
The development of macular edema and visual change resulting from vein occlusion is thought to be at least in part mediated by the release of vascular endothelial growth factor. As the use of anti-VEGF agents such as bevacizumab and ranibizumab has gained more widespread favor for conditions other than age-related macular degeneration, many reports have entered the literature regarding the use of these agents for the treatment of vein occlusions. Retrospective analysis seems to indicate a potential role for anti-VEGF agents.11-16 Short-term, randomized prospective data on the efficacy of these agents for treatment of branch or central retinal vein occlusion are described below.
Six-month randomized prospective data from the BRAVO and CRUISE trials comparing ranibizumab 0.3 mg vs. ranibizumab 0.5 mg vs. sham injection in BRVO and CRVO have also supported a potential role for anti-VEGF agents in the treatment of vein occlusion. The six-month data from the BRAVO trial showed that 55.2 percent of patients (74 of 134) who received 0.3 mg of ranibizumab and 61.1 percent of patients (80 of 131) who received 0.5 mg of ranibizumab gained 15 letters of BCVA compared with 28.8 percent of patients (38/132) who received sham injections. Likewise, six-month data from the CRUISE trial showed that 46.2 percent of patients (61/132) who received 0.3 mg of ranibizumab and 47.7 percent of patients (62/130) who received 0.5 mg of ranibizumab gained 15 letters or more compared with 16.9 percent of patients (22/130) who received sham injections.
While both trials do suggest at least short-term efficacy of anti-VEGF injections over sham injection, long-term randomized prospective data is not yet available. The BRAVO trial did not compare intravitreal ranibizumab injections to macular grid photocoagulation.17,18
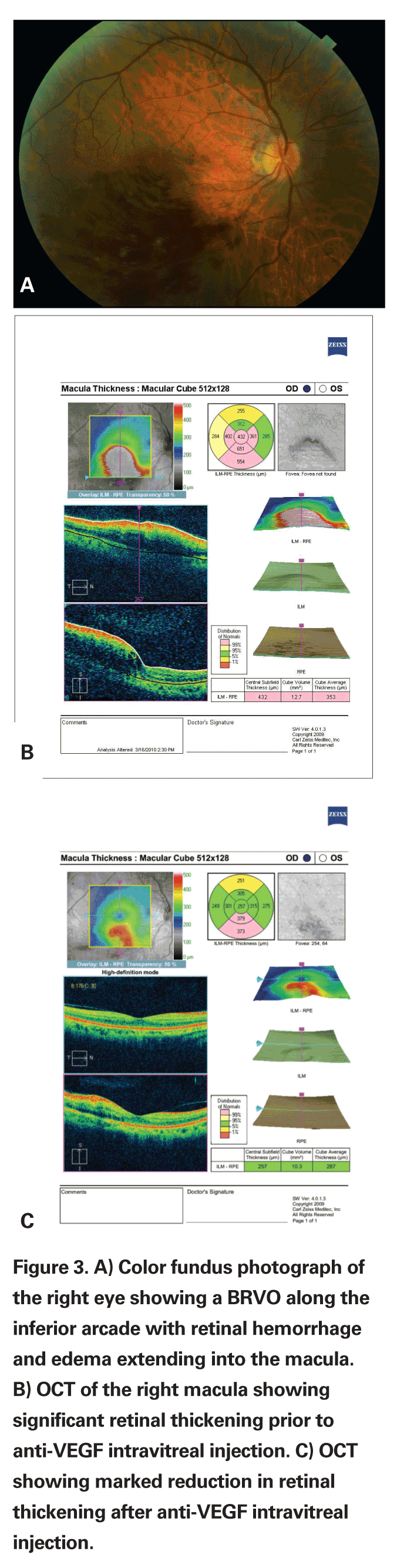
While these reports seem to indicate that anti-VEGF injections do not tend to induce rises in intraocular pressure necessitating the use of IOP-lowering medications, or induce cataract formation, long-term data is still unavailable. Regardless, intravitreal injections of anti-VEGF agents are still subject to concerns regarding both sterile and infectious endophthalmitis.
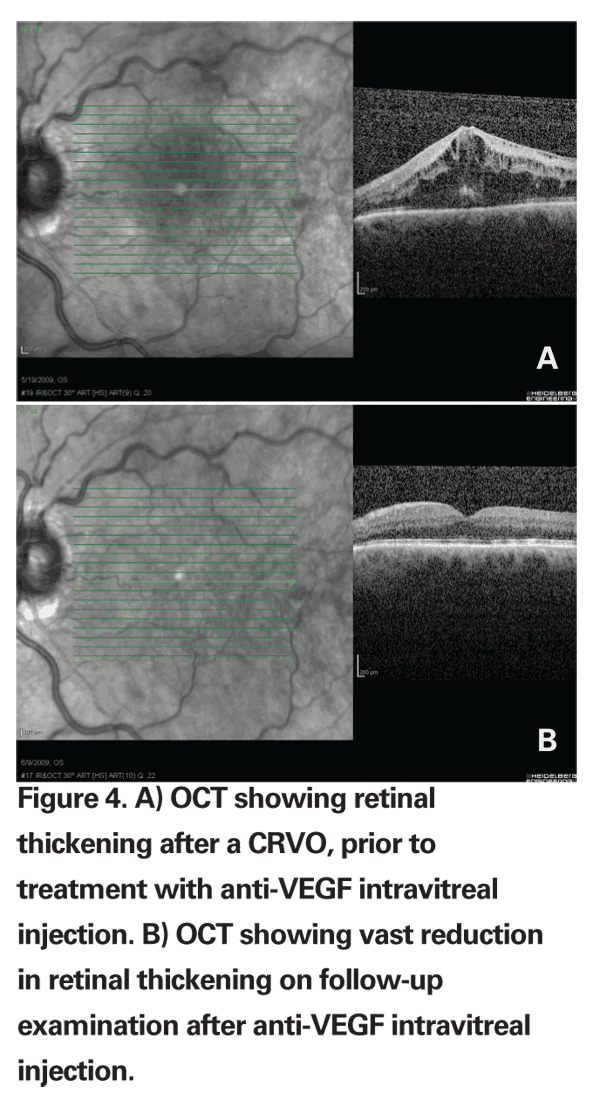
Advances in the development of different medications targeted for the treatment of ocular vascular dysfunction has changed the landscape of modern retinal practice. Macular grid photocoagulation, intravitreal anti-VEGF agents and intravitreal triamcinolone are all used widely for macular edema secondary to branch retinal vein occlusion and central retinal vein occlusion. It will be interesting to note if the promising results from the BRAVO and CRUISE trials spur future trials comparing intravitreal anti-VEGF agents to macular grid photocoagulation and intravitreal triamcinolone for the treatment for branch and central retinal vein occlusions.
Dr.Youssef is a vitreoretinal surgeon at the Eye Clinic of Wisconsin in
1. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol 1984;98:271-82.
2. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995;102:1425-33.
3. Greenberg PB, Martidis A, Rogers AH, Duker JS, Reichel E. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol 2002;86:247-8.
4. Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol 2004;122:1131-6.
5.Ip MS,
6. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefe's Arch Clin Exp Ophthalmol 2002;240:782-3.
7. Wakabayashi T, Okada AA, Morimura Y, Kojima E, Asano Y,
8. Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol 2009;127:1115-28.
9. Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol 2009;127:1101-14.
10. Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: Progress to date. Drugs Aging 2010;27(2):117-34.
11. Abegg M, Tappeiner C, Wolf-Schnurrbusch U, Barthelmes D, Wolf S, Fleischhauer J. Treatment of branch retinal vein occlusion induced macular edema with bevacizumab. BMC Ophthalmol 2008;8:18.
12. Badala F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol 2008;19(3):234-8.
13.
14. Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina 2006;26:279-84.
15. Priglinger SG, Wolf AH, Kreutzer TC, Kook D, Hofer A, Strauss RW, et al. Intravitreal bevacizumab injections for treatment of central retinal vein occlusion: Six-month results of a prospective trial. Retina 2007;27:1004-12.
16. Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007;27:419-25.
17. Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for Macular Edema following Central Retinal Vein Occlusion Six-Month Primary End Point Results of a Phase III Study. Ophthalmology 2010;117:1061-3.
18. Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for Macular Edema following Branch Retinal Vein Occlusion Six-Month Primary End Point Results of a Phase III Study. Ophthalmology. 2010 Jun;117(6):1102-1112.e1. Epub 2010 Apr 15.



