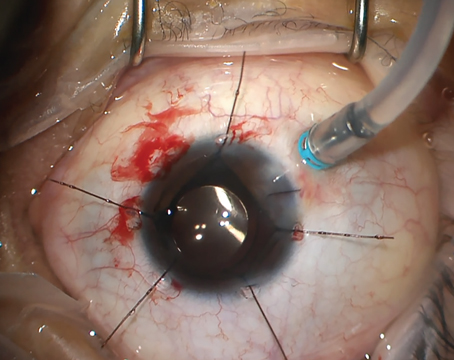
Though most macular holes can be closed successfully, it’s the complicated hole presentations that can test your skills. Fortunately, surgeons and researchers have identified the factors that make MH closure more difficult, as well as techniques that can assist you in closing these more challenging holes. To benefit from their insights, read on.
What Makes a MH Complex
Macular holes can cause significantly decreased vision and symptomatic central scotomas.1 Surgical repair with pars plana vitrectomy, inner limiting membrane peeling and gas tamponade yields closure rates of greater than 90 percent.2,3 However, certain risk factors for unsuccessful hole closure have been identified, including large size, myopic MHs, chronic MHs, traumatic MHs, concurrent retinal detachment, and previous unsuccessful macular hole surgery (refractory MHs).2,4,5
Several techniques have been developed to increase macular hole surgery success rates in cases with the previously listed risk factors. Some of these techniques include:
- additional conventional surgical methods (broader ILM peeling and repeat fluid-gas exchange);
- increasing retinal tissue compliance (macular detachment, retinal incisions);
- MH scaffolds (inverted ILM flap, ILM free flap, posterior capsule flap);
- the use of growth factors/cytokines to aid healing (placement of autologous blood/platelet rich plasma [PRP] or transforming growth factor-beta 2 [TGFβ2] into the macular hole, and macular laser);
- pre- and subretinal amniotic membrane (AM) placement in the MH to act as both a scaffold and to release growth factors/cytokines to promote healing; and
- tissue replacement (autologous neurosensory retinal transplant).
Next, we’ll delve into each of these techniques in greater detail.
Additional Conventional Surgical Methods
Re-staining with indocyanine green or brilliant blue dye may identify an area of ILM that was missed during the initial peel, or reveal that a relatively small amount of ILM was initially peeled. Enlarging the previous ILM peel results in closure in 47 to 69 percent of refractory MH cases through further release of tangential traction on the hole (Figure 1).6,7 However, one study found that even in cases with anatomic improvement, there was limited visual improvement.6 Studies have also found that performing a fluid-gas exchange in the clinic for refractory or reopened MHs resulted in a 74 to 89 percent closure rate and improved vision.8,9 A benefit of this technique is that it avoids another trip to the operating room.
Increasing Retinal Tissue Compliance
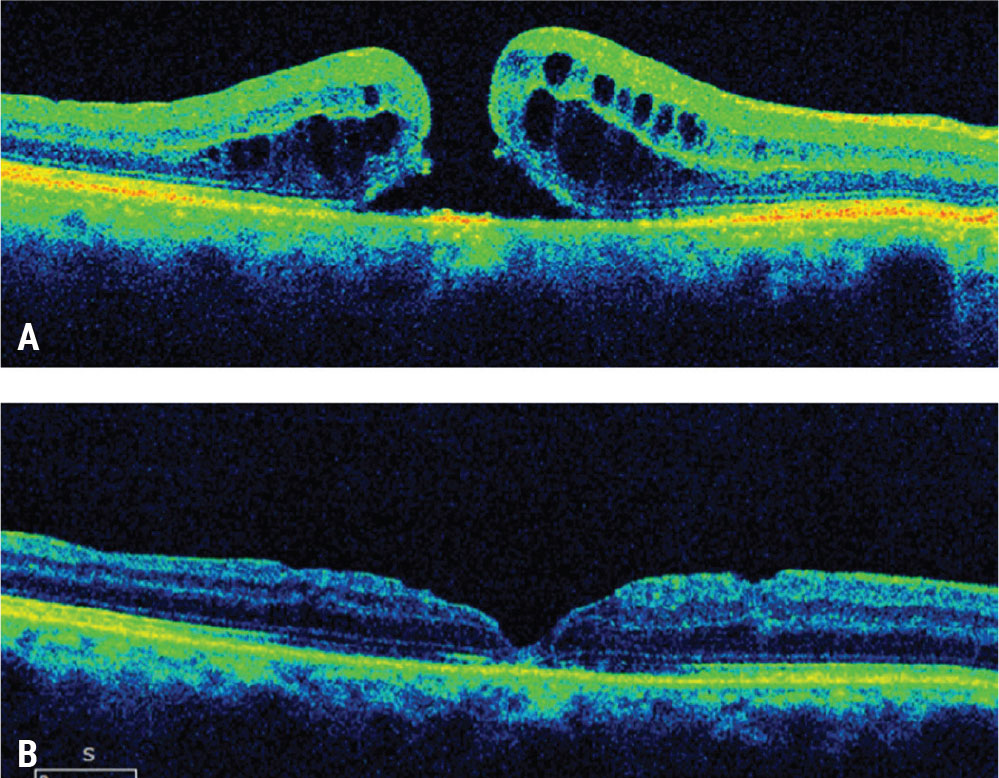 |
| Figure 1. Wider inner limiting membrane peeling for a refractory macular hole. This patient presented with a visual acuity of 20/100 a month after failed initial macular hole surgery with ILM peeling (A). A subsequent wider ILM peel was performed with repeat gas tamponade. The macular hole closed with visual acuity improving to 20/30 (B). Click image to enlarge. |
There are multiple approaches that can be employed to increase retinal tissue compliance in order to increase the likelihood of closing a complex MH.
One such approach is induction of a macular detachment.10 Small-gauge (e.g., 38-gauge) cannulas connected to the viscous fluid injection (VFI) kit can be used to perform controlled subretinal injection of BSS within the arcades to create blebs of subretinal fluid contiguous with the macular hole in all quadrants. A subsequent fluid-air exchange followed by gas tamponade has been reported to yield a 90-percent closure rate, with visual improvement in refractory or recurrent MHs (Figure 2).11 In order to avoid creating additional entry sites into the retina, the University of Toronto’s Tina Felfeli, MD, and Efrem Mandelcorn, MD, developed a modification of this technique: A silicone soft-tip extrusion cannula is used to reflux fluid through the macular hole into the subretinal space.12 The edges of the macular hole are then brushed together using passive extrusion, followed by a fluid-air exchange to drain any residual subretinal fluid through the hole, and then gas tamponade (Figure 3). In a series of 39 complex (refractory, chronic, or ≥400 µm diameter) holes, this technique resulted in a 95 percent closure rate with vision improvement in 95 percent of patients.12 Of note, these techniques are especially helpful in post-traumatic MH cases in which there is chorioretinal scarring in the extrafoveal macula that’s limiting the tissue mobility.
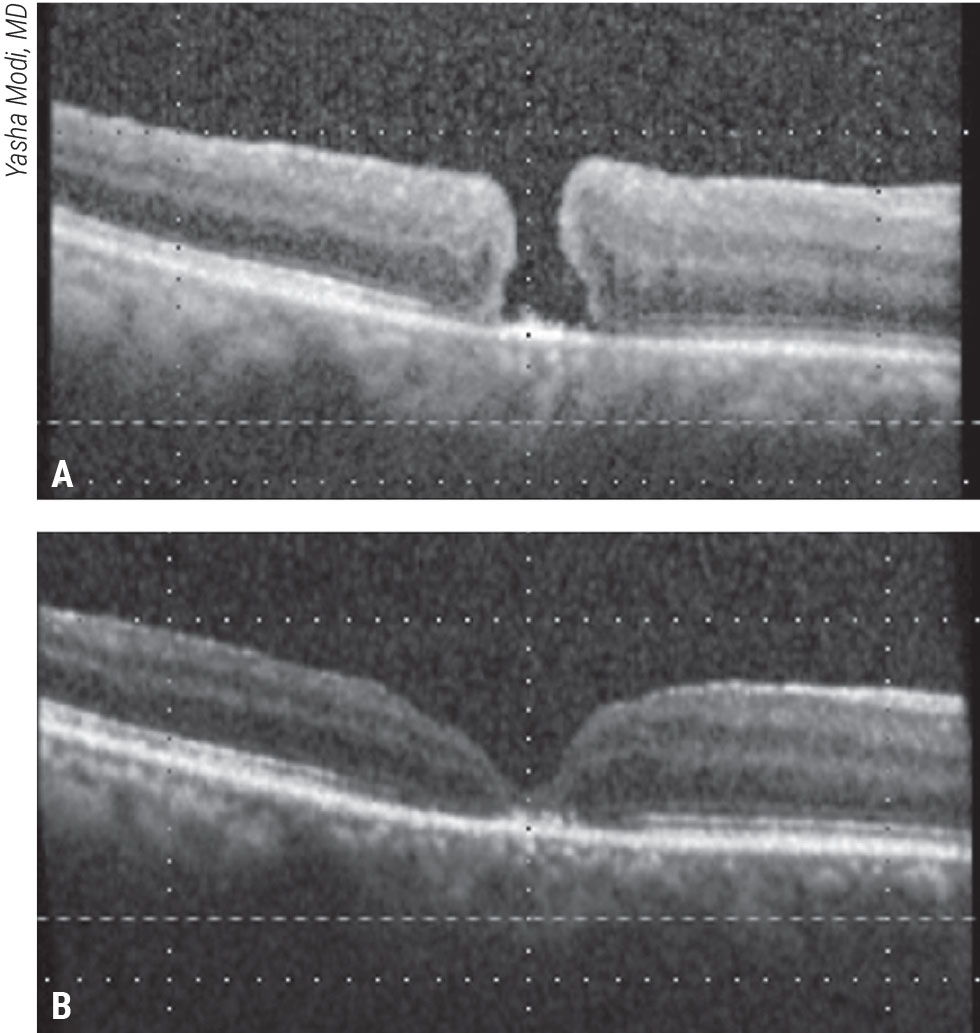 |
Figure 2. Macula detachment for a refractory macular hole. This patient presented with a visual acuity of 20/400 two weeks after failed initial macular hole surgery with ILM peeling (A). A macular detachment was induced, followed by fluid-air exchange and gas tamponade. The macular hole closed and vision improved to 20/150 (B). Click image to enlarge. |
Performing arcuate or radial full-thickness retinal incisions is an alternative approach to increasing retinal tissue compliance. Germantown, Tennessee, surgeon Steve Charles and colleagues first described the following arcuate retinotomy technique, which consists of an arcuate full-thickness incision 500 to 700 µm temporal to the MH, followed by gas tamponade.13 In six patients with large, refractory MHs this technique yielded an 83 percent MH closure rate and half of patients had improved vision.13 Oporto, Portugal, surgeon Rita Reiss and colleagues described a variation of this technique in which the surgeon creates five radial full-thickness incisions centered on the MH, and extends them one hole diameter away from the MH border. This is followed by fluid-air exchange and gas tamponade.14 Using this technique, the investigators reported a 100-percent anatomic success rate with a mean gain of 5.6 lines of vision in seven patients with refractory MHs.14
While the techniques outlined above have demonstrated efficacy, the need to create a full-thickness neuroretinal incision—without causing any damage to the underlying retinal pigment epithelium and the choroid—makes these approaches more surgically challenging than some of the other techniques for complex MHs.
MH Scaffolds
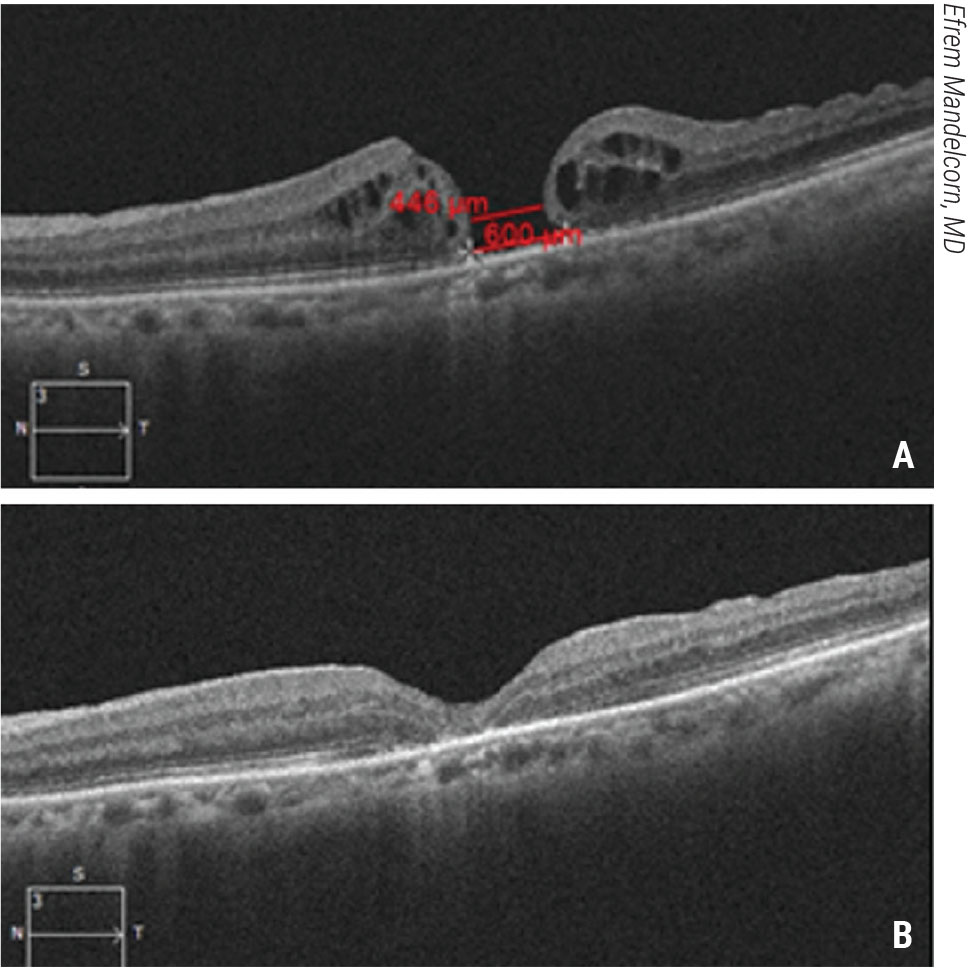 |
| Figure 3. Macular hydrodissection for a large macular hole. This patient presented with a large macular hole and visual acuity of 20/200 (A). Vitrectomy and macular hydrodissection with a backflush cannula and gas tamponade were performed. The patient’s macular hole closed and vision improved to 20/80 (B). Click image to enlarge. |
Another approach for complex MHs is to provide a scaffold for Müller cell and tissue proliferation within the hole to aid in its closure. There are several techniques for accomplishing this, including the inverted ILM flap, ILM free flap and lens capsule flap techniques. A benefit of ILM flaps vs. capsule flaps is that, in addition to providing a scaffold, the ILM tissue may contain Müller cell fragments, which have been hypothesized to induce gliosis, which may increase rates of MH closure.4,15,16 The flaps also provide a “lid” to the macular hole that the retinal pigment epithelium can pump against, to help with closing the hole.
Zofia Michalewska, MD, and her colleagues in Lodz, Poland, first described the inverted ILM flap technique for large macular holes.15 In this technique, a wide ILM peel is completed with careful attention to detaching the peripheral macular ILM, while maintaining the ILM attachment around the MH.15 Subsequently, the detached ILM is inverted and placed into the MH, followed by fluid-air exchange.15 This approach resulted in a higher rate of MH closure in patients (98 percent) compared to traditional MH surgery (88 percent), along with better visual outcomes (Figure 4).15 In a multicenter series comparing the outcomes of traditional ILM peeling and the inverted ILM flap technique for holes ≥800 µm in diameter, surgeons found non-statistically significantly improved hole closure (89 percent vs. 78 percent) and better vision recovery using the inverted ILM flap technique.17 A larger study of this technique found that MHs ≥400 µm in diameter had significantly better closure rates using the inverted ILM flap technique (96 percent) compared to conventional ILM peeling (79 percent), with better visual outcomes.
Several variations of the inverted ILM flap technique have been reported, with encouraging anatomic and visual outcomes. One such technique, the temporal inverted ILM flap technique, involves only peeling the ILM temporal to the MH, while keeping the edges of the ILM attached to the MH, and then draping the temporal ILM flap over the MH.18 The opposite approach has also been described and named the “Texas taco” technique, in which the nasal ILM is peeled just beyond the temporal edges of the hole and then the ILM flap is draped over the MH.19
While the approaches discussed above are useful for cases that haven’t previously undergone ILM peeling, refractory MHs that didn’t close after ILM peeling aren’t amenable to them. Okayama, Japan’s Yuki Morizane, MD, co-authored a study that described the use of a free ILM flap to provide a MH scaffold in cases with a prior complete macular ILM peel (Figure 5).20 They used this technique in 10 patients with refractory MHs and achieved 90-percent anatomic closure and visual improvement.20 Additional studies of this technique in a small series of refractory MH patients21 and complex MH patients (large, chronic, and refractory MHs) found similar results.22
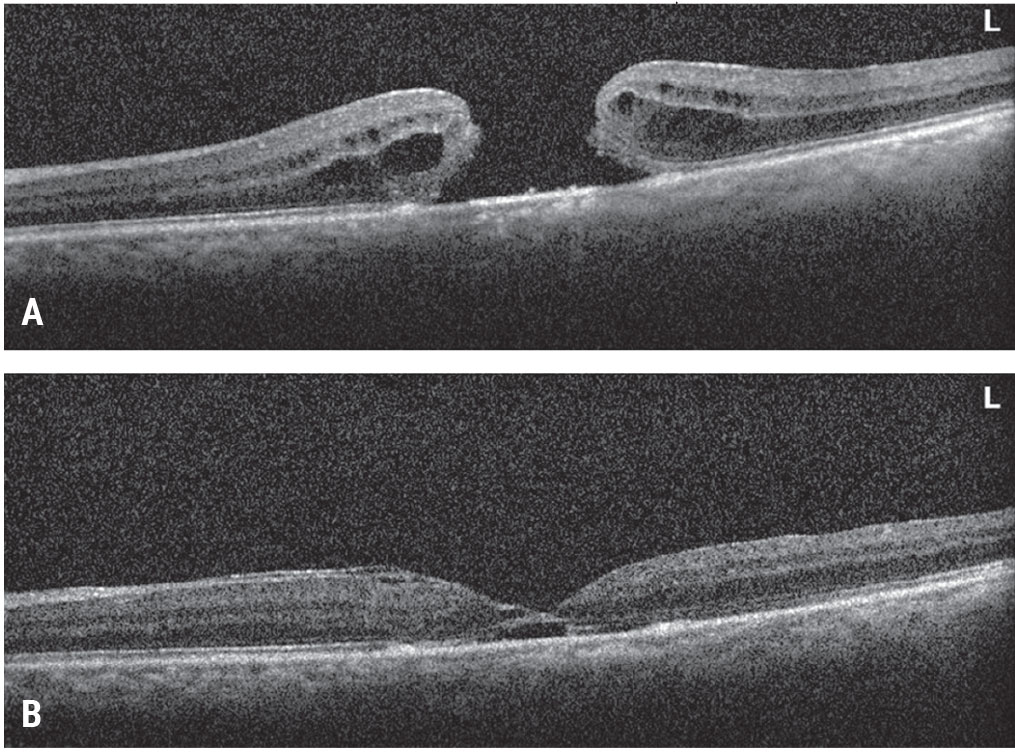 |
| Figure 4. Inverted ILM flap for a large macular hole. This patient presented with a large macular hole and visual acuity of 20/200 (A). Vitrectomy, inverted ILM flap, fluid-air exchange and gas tamponade were performed. The patient’s macular hole closed and vision improved to 20/70 (B). Click image to enlarge. |
In patients who have had a very wide ILM peel, the residual peripheral ILM may be thin and friable, rendering it difficult to obtain a suitable free flap. In such cases the use of non-ILM tissue, such as lens capsule, has been reported to be effective at accomplishing anatomic closure, and leading to improved vision.23 One of the most challenging aspects of this technique is maintaining the free ILM or lens capsule flap in the macular hole during the fluid-air exchange. Viscoelastic agents or perfluorocarbon (PFC, Perfluoron, Alcon Laboratories) can help maintain the flap in position and limit the redundancy of the flap in the MH.24,25 Alternatively, chandelier illumination with a bimanual technique can be used to hold the flap in place with the forceps while performing the fluid-air exchange (Figure 6).
Growth Factors and Cytokines
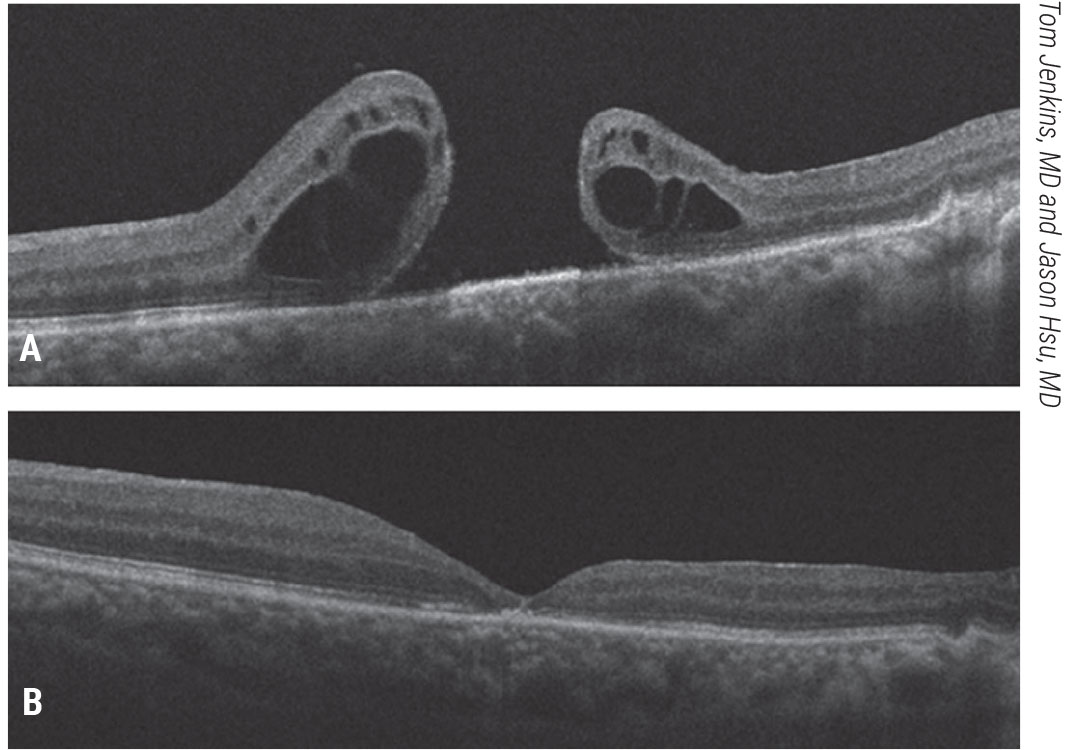 |
| Figure 5. Free inner limiting membrane flap for a refractory, traumatic macular hole. A patient with a history of a traumatic macular hole who underwent vitrectomy, ILM peeling and gas tamponade presented with a refractory macular hole and 20/200 vision (A). He underwent ILM free flap placement into the macular hole, fluid-air exchange and gas tamponade. His macular hole closed and vision improved to 20/50 (B).Click image to enlarge. |
Since growth factors and cytokines modulate the physiologic wound healing response and may aid MH closure,26 one approach to complex MHs involves placing these adjuvants into the MH. Bascom Palmer surgeon William Smiddy and his colleagues found that using TGFβ2 for refractory MHs led to an 83-percent closure rate with ≥3 lines of vision in 52 percent of patients.27 Another prospective study utilizing this technique found a dose-response effect with further vision improvement with higher concentrations of TGFβ2.28 The lack of commercially available and FDA-approved TGFβ2 for intraocular use has limited the use of this technique, however.
TGFβ2 is one of several growth factors and cytokines released by platelets. Platelet-rich plasma has been used in various fields of medicine to modulate wound healing.29 For complex MHs, autologous PRP has been studied in myopic and refractory MHs with high closure rates and improved vision.30,31 Obtaining PRP from a patient’s peripheral blood requires special equipment, which may not be readily available at all surgical facilities, so others have looked at the use of autologous blood to aid MH closure. One study found low rates of closure in refractory MHs with autologous blood,31 but another found high rates of closure when combining the inverted ILM flap technique and autologous blood for large MHs.32
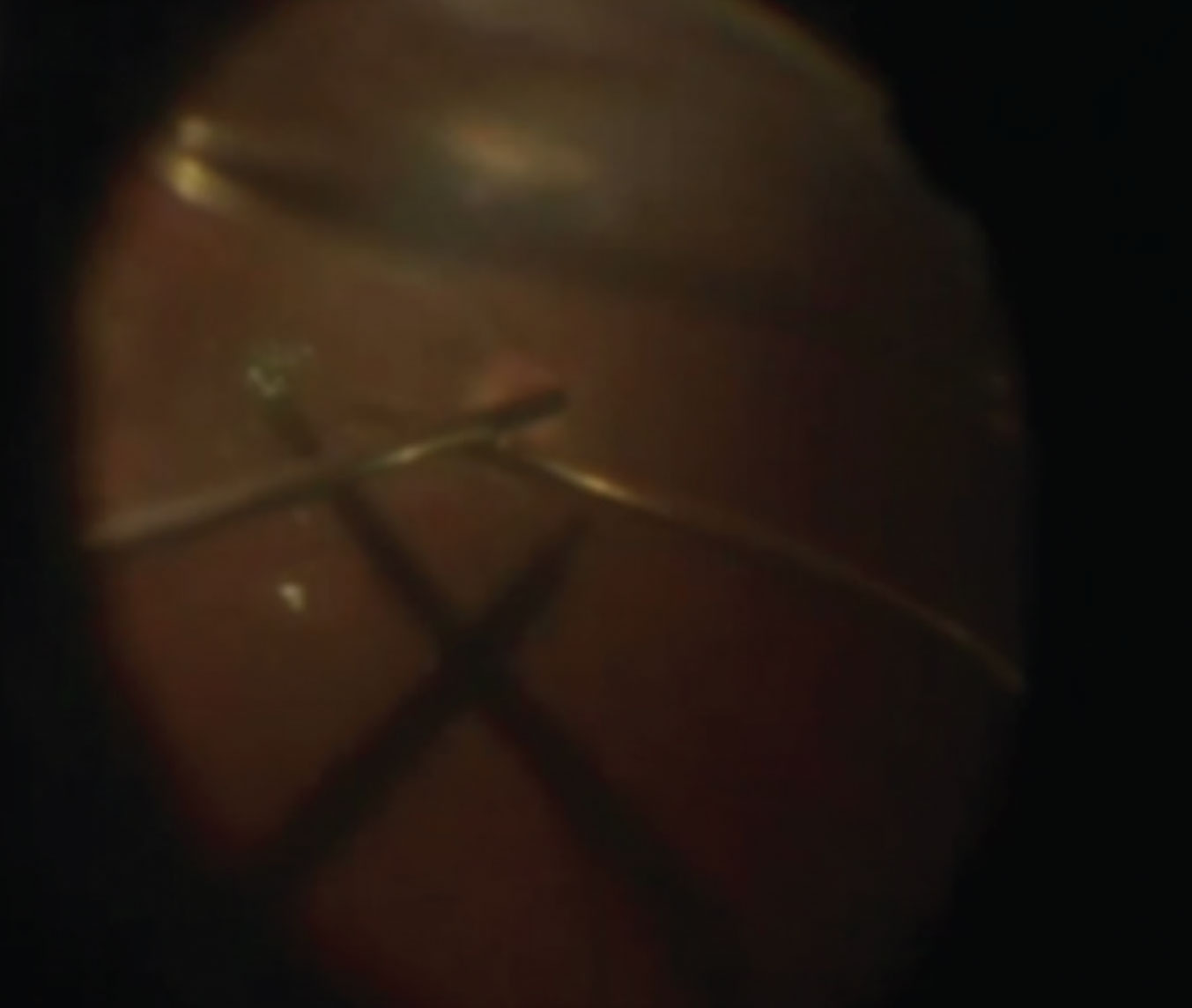 |
| Figure 6. Bimanual technique for holding a free ILM flap in place. Chandelier illumination facilitates the bimanual technique of holding the ILM free flap over the macular hole during fluid-air exchange (see partial air fill at the top of the image). Click image to enlarge. |
Laser can induce the release of growth factors and cytokines without the placement of adjuvants into the eye. One study compared outcomes after application of laser photocoagulation at the center of MH prior to vitrectomy (three burns of 100 µm size, 0.04- to 0.1-second duration, and 60 to 100 mW), followed by vitrectomy, ILM peeling and gas tamponade (94%), versus traditional vitrectomy, ILM peeling and gas tamponade without pre-surgical laser photocoagulation, in large MHs (≥400 µm diameter).33 There were higher MH closure rates and significantly better visual outcomes in the complex MH patients who underwent the preoperative laser treatment.33
The presence of lasers in most retina clinics makes this an easily accessible technique. While there are concerns about creating a scotoma from laser in the macula, the settings are low enough that a visually significant scotoma is unlikely.
Amniotic Membrane
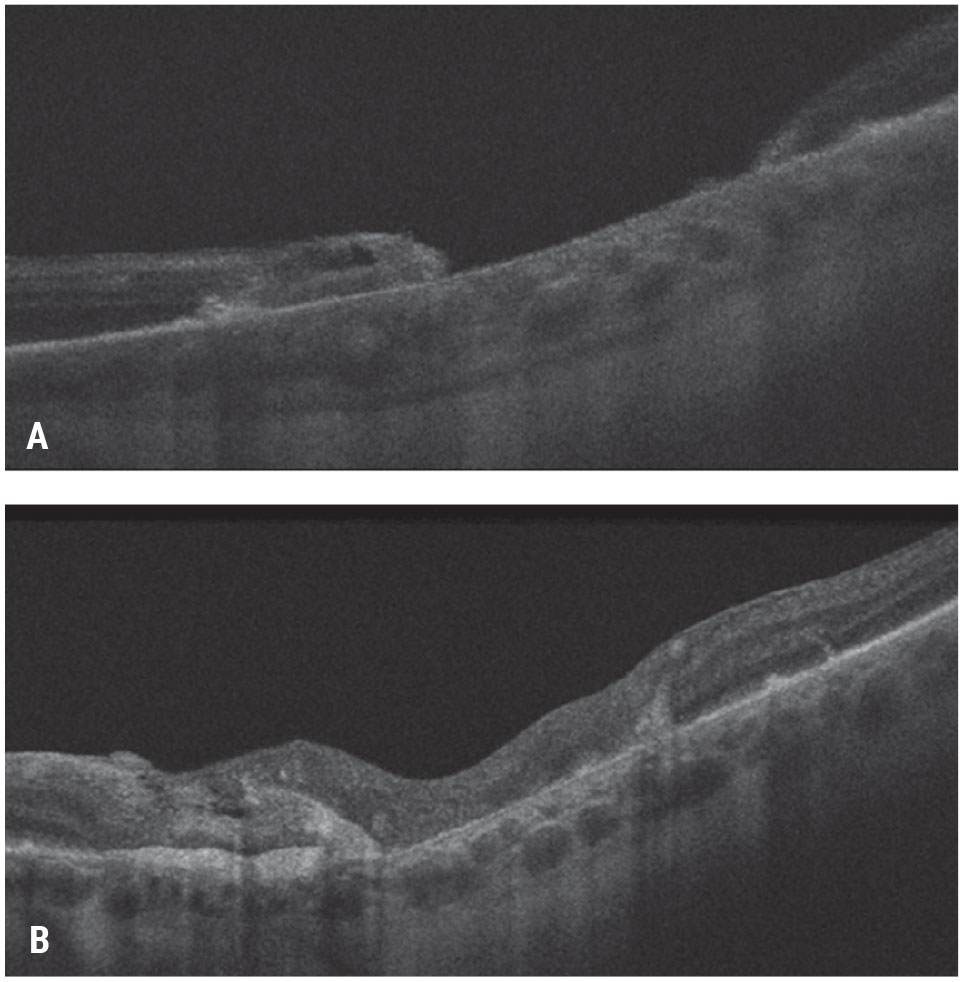 |
| Figure 7. Autologous neurosensory retinal transplant for a large traumatic macular hole. This patient had a large traumatic macular hole with underlying choroidal atrophy secondary to an intraocular foreign body in the macula and count-finger vision (A). The patient underwent vitrectomy, inner limiting membrane peeling, autologous retina transplant into the macular hole, silicone oil tamponade for three months and silicone oil removal. The patient’s hole closed with visual acuity improving to 20/200-1 (B). Click image to enlarge. |
An approach that combines the goals of providing a scaffold and modulating wound healing is the placement of amniotic membrane in the MH. AM has been demonstrated to be non-toxic to the retina in animal studies, acts as a scaffold for wound healing, and releases several factors that promote wound healing.5,34,35 Stanislao Rizzo, MD, and colleagues at the University of Florence first described placement of subretinal human AM (obtained from a tissue bank), followed by gas tamponade, in eight patients with refractory MHs, which yielded 100-percent closure and improvement of vision.36 One study reported the use of commercially available human amniograft from Bio-Tissue (Tissue Tech, Miami) using sub- and pre-retinal placement approaches (Figure 7 and 8) resulting in successful hole closure and improved visual outcomes.5 For this technique, a dermal punch can be used to create the appropriately sized tissue for hole placement. It’s important that the chorion (“sticky”) side is facing the retinal pigment epithelium. Forceps can be used to grasp the non-chorion side and fold the AM to facilitate insertion into the vitreous cavity through a cannula. Cutting a small notch on the AM can aid the orientation of the membrane inside the eye. Once placed in or over the MH, the AM is much more adherent and is less likely to move during the fluid-air exchange than the ILM or capsule free flaps.
Tissue Replacement
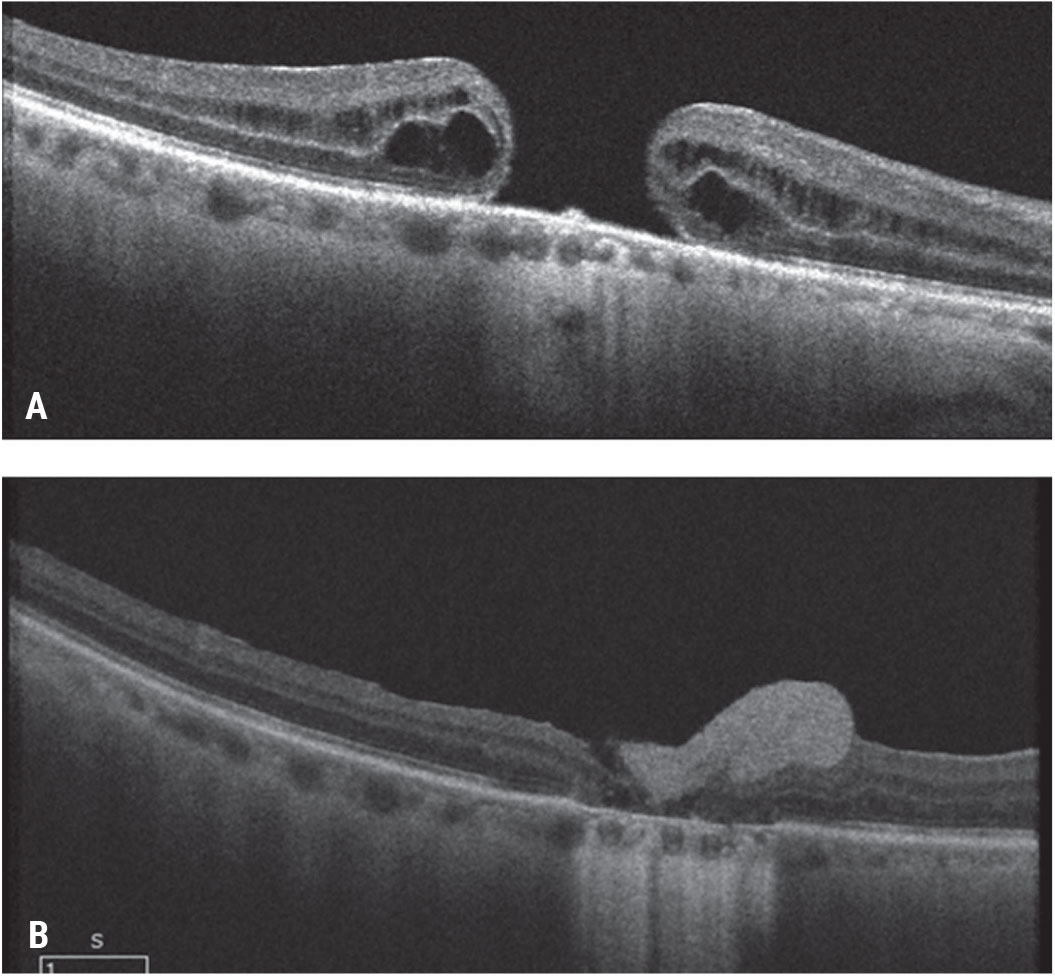 |
| Figure 8. Pre-retinal human amniotic membrane for a chronic, large macular hole. A patient presented with a chronic macular hole of more than two years’ duration after a prior retinal detachment repair and count fingers vision (A). He underwent vitrectomy, ILM peeling and pre-retinal placement of human amniotic membrane over the macular hole and gas tamponade. As the amniotic membrane dissolved (hyperreflective preretinal material), the hole closed and the visual acuity improved to 20/150 (B). Click image to enlarge. |
Placement of peripheral autologous retina into the macular hole has shown some efficacy in refractory MHs.37 In this technique, a patch of retina is cut from the periphery of the eye and placed over the hole. The study reported an 88-percent closure rate and significantly improved vision in these patients (Figure 9).37 This technique has also been successful in myopic MHs.38 The use of PFO prior to the creation of the peripheral autologous graft is very helpful. The PFO should be instilled until it covers the retina peripheral to the location of the planned graft harvest site. It’s recommended that the retina graft be half a disc diameter larger than the size of the MH.37 This area can be marked by diathermy, followed by laser around the site. The graft can then be created with pneumatic vertical scissors and moved under the PFO to the hole. The use of fluid-air exchange and then silicone oil or gas placement, direct PFO to silicone oil exchange, and simply short term PFO tamponade have all been described.38
In conclusion, traditional MH surgery is generally successful, but complex MHs can have lower success rates. There are multiple options for these complex MHs that have been found to have positive anatomic and visual outcomes. At this point, there’s still a paucity of data comparing these techniques, and no single technique has been found to be superior.
Dr. Kuriyan is an assistant professor of ophthalmology at Sidney Kimmel Medical College of Thomas Jefferson University. He has no relevant disclosures.
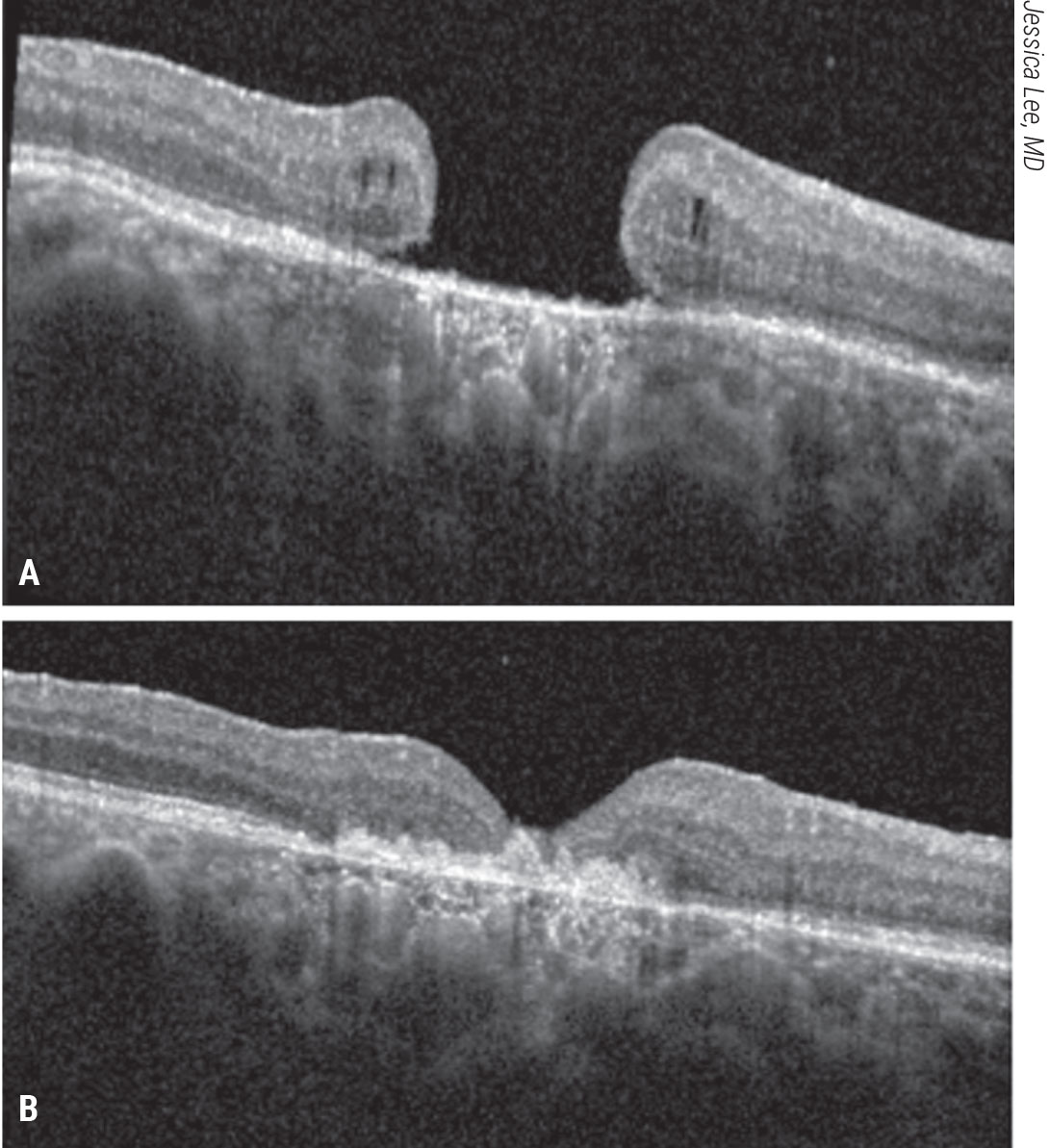 |
| Figure 9. Subretinal human amniotic membrane for a refractory macular hole. A patient with count-fingers vision presented with a refractory macular hole after prior vitrectomy, ILM peeling and gas tamponade. A human amniotic membrane graft was placed in a subretinal position followed by gas tamponade. The patient’s macular hole closed and visual acuity improved to 20/200 (B). Click image to enlarge. |
1. McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology 2009;116:7:1366–9.
2. Kumagai K, Furukawa M, Ogino N, Uemura A, Demizu S, Larson E. Vitreous surgery with and without internal limiting membrane peeling for macular hole repair. Retina 2004;24:5:721–7.
3. Sheidow TG, Blinder KJ, Holekamp N, Joseph D, Shah G, Grand MG, et al. Outcome results in macular hole surgery: An evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology 2003;110:9:1697–701.
4. Grewal DS, Fine HF, Mahmoud TH. Management of challenging macular holes: Current concepts and new surgical techniques. Ophthalmic Surg Lasers Imaging Retina 2016;47:6:508–13.
5. Kuriyan AE, Hariprasad SM, Fraser CE. Approaches to refractory or large macular holes. Ophthalmic Surg Lasers Imaging Retina 2020;51:7:375–82.
6. D’Souza MJ, Chaudhary V, Devenyi R, Kertes PJ, Lam W-C. Re-operation of idiopathic full-thickness macular holes after initial surgery with internal limiting membrane peel. Br J Ophthalmol 2011;95:11:1564–7.
7. Moisseiev E, Fabian ID, Moisseiev J, Barak A. Outcomes of repeated pars plana vitrectomy for persistent macular holes. Retina 2013;33:6:1137–43.
8. Johnson RN, McDonald HR, Schatz H, Ai E. Outpatient postoperative fluid—gas exchange after early failed vitrectomy surgery for macular hole. Ophthalmology 1997;104:12:2009–13.
9. Rao P, Lum F, Wood K, Salman C, Burugapalli B, Hall R, et al. Real-world vision in age-related macular degeneration patients treated with single anti–VEGF drug type for 1 year in the IRIS Registry. Ophthalmology 2018;125:4:522-528.
10. Oliver A, Wojcik EJ. Macular detachment for treatment of persistent macular hole. Ophthalmic Surg Lasers Imaging Retina 2011;42:6:516–8.
11. Szigiato A-A, Gilani F, Walsh MK, Mandelcorn ED, Muni RH. Induction of macular detachment for the treatment of persistent or recurrent idiopathic macular holes. Retina 2016;36:9:1694–8.
12. Felfeli T, Mandelcorn ED. MACULAR hole hydrodissection: Surgical technique for the treatment of persistent, chronic, and large macular holes. Retina 2019;39:4:743–52.
13. Charles S, Randolph JC, Neekhra A, Salisbury CD, Littlejohn N, Calzada JI. Arcuate retinotomy for the repair of large macular holes. Ophthalmic Surg Lasers Imaging Retina 2013;44:1:69–72.
14. Reis R, Ferreira N, Meireles A. Management of stage IV macular holes: When standard surgery fails. Case Rep Ophthalmol 2012;3:2:240–50.
15. Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010;117:10:2018–25.
16. Singh SR, Hariprasad SM, Narayanan R. Current management of macular hole. Ophthalmic Surg Lasers Imaging Retina 2019;50:2:61–8.
17. Narayanan R, Singh SR, Taylor S, Berrocal MH, Chhablani J, Tyagi M, et al. Surgical outcomes after inverted internal limiting membrane flap versus conventional peeling for very large macular holes. Retina 2019;39:8:1465–9.
18. Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: A comparative study. Retina 2015;35:9:1844–50.
19. Major JJ, Lampen SI, Wykoff CC, Ou WC, Brown DM, Wong TP, et al. The Texas taco technique for internal limiting membrane flap in large full-thickness macular holes: A Short-Term Pilot Study. Retina 2020;40:3:552-556.
20. Morizane Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, Kawata T, et al. Autologous transplantation of the internal limiting membrane for refractory macular holes. Am J Ophthalmol 2014;157:4:861–9.
21. Pires J, Nadal J, Gomes NL. Internal limiting membrane translocation for refractory macular holes. Br J Ophthalmol 2017;101:3:377–82.
22. De Novelli FJ, Preti RC, Monteiro MLR, Pelayes DE, Nóbrega MJ, Takahashi WY. Autologous internal limiting membrane fragment transplantation for large, chronic, and refractory macular holes. Ophthalmic Res 2016;55:1:45–52.
23. Chen S-N, Yang C-M. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina 2016;36:1:163–70.
24. Andrew N, Chan WO, Tan M, Ebneter A, Gilhotra JS. Modification of the inverted internal limiting membrane flap technique for the treatment of chronic and large macular holes. Retina 2016;36:4:834–7.
25. Shin MK, Park KH, Park SW, Byon IS, Lee JE. Perfluoro-n-Octane–assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina 2014;34:9:1905–10.
26. Idrees S, Sridhar J, Kuriyan AE. Proliferative Vitreoretinopathy: A Review. Int Ophthalmol Clin 2019;59:1:221–40.
27. Smiddy WE, Sjaarda RN, Glaser BM, Flynn JH, Thompson JT, Hanham A, et al. Reoperation after failed macular hole surgery. Retina 1996;16:1:13–8.
28. Glaser BM, Michels RG, Kuppermann BD, Sjaarda RN, Pena RA. Transforming growth factor-β2 for the treatment of full-thickness macular holes: A prospective randomized study. Ophthalmology 1992;99:7:1162–73.
29. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009;37:11:2259–72.
30. Figueroa MS, Govetto A, de Arriba-Palomero P. Short-term results of platelet-rich plasma as adjuvant to 23-G vitrectomy in the treatment of high myopic macular holes. Eur J Ophthalmol 2016;26:5:491–6.
31. Purtskhvanidze K, Frühsorger B, Bartsch S, Hedderich J, Roider J, Treumer F. Persistent full-thickness idiopathic macular hole: Anatomical and functional outcome of revitrectomy with autologous platelet concentrate or autologous whole blood. Ophthalmologica 2018;239:1:19–26.
32. Lyu W-J, Ji L-B, Xiao Y, Fan Y-B, Cai X-H. Treatment of refractory giant macular hole by vitrectomy with internal limiting membrane transplantation and autologous blood. Int J Ophthalmol 2018;11:5:818.
33. Cho HY, Kim YT, Kang SW. Laser photocoagulation as adjuvant therapy to surgery for large macular holes. Korean J Ophthalmol 2006;20:2:93–8.
34. Rosenfeld PJ, Merritt J, Hernandez E, Meller D, Rosa RH, Tseng SCG. Subretinal implantation of human amniotic membrane: A rabbit model for the replacement of Bruch’s membrane during submacular surgery. Invest Ophthalmol Vis Sci 1999;40:4:S206–S206.
35. Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, et al. How does amniotic membrane work? Ocul Surf 2004;2:3:177–87.
36. Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:S95–103.
37. Grewal DS, Charles S, Parolini B, Kadonosono K, Mahmoud TH. Autologous retinal transplant for refractory macular holes: Multicenter international collaborative study group. Ophthalmology 2019;126:10:1399-1408.
38. Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol 2016;134:2:229–30.