As the number of Descemet’s membrane endothelial keratoplasty procedures performed increases, so do technique modifications and advances. Many of these methods aim at simplifying certain steps and softening the learning curve; others adapt the technique for complex eyes. Here, surgeons share their new DMEK techniques and offer some tips for achieving good outcomes.
Orientation
While “S” and “F” stamps can serve as final checks to ensure proper anterior-posterior orientation, some experts believe that stamping grafts with ink, and the extra manipulation that entails, may not be the future of DMEK. A study of 31 DMEK grafts reported that tissue preloading resulted in greater endothelial cell loss than pre-stripping tissue;1 another reported complete loss of cells in the S-stamped area.2
Here are some of the ways surgeons are ensuring proper orientation without stamping:
• Double-scrolling. A donor Descemet’s membrane can take on several different configurations, but according to Rajesh Fogla, DNB, FRCSEd, FRCOphth, FACS, of the Eye Department of Apollo Hospitals in Jubilee Hills, Hyderabad, India, a double-scroll configuration is the most ideal. “A double-scroll configuration allows for donor insertion in the correct orientation, i.e., the open end of the scroll faces upwards, thereby requiring minimal manipulation over a shorter period of time,” he says. “This helps to minimize intraoperative donor endothelial cell loss and provide better DMEK outcomes.”
His DMEK technique involves two modifications.3 First, he creates a double-scroll orientation prior to graft insertion. To do this, the stained donor scroll is aspirated into a Geuder injector. This is followed by agitation of the fluid column, moving the donor scroll between the narrow and wide portions of the glass injector. “As the donor scroll moves from the narrow to the wide portion, it opens up and scrolls back due to the tissue’s elasticity,” he explains. “Repeating this five to six times results in a double-scroll configuration. We then aspirate the double scroll into the narrow portion of the injector to retain its configuration. We were able to achieve a double-scroll configuration in 85 percent of cases in our study (n=20 eyes).”
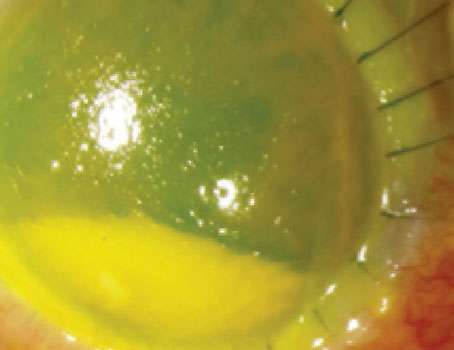
 |
| Figure 1. Unscrolling the double-scrolled graft with surface tapping. |
The second modification involves inserting the double-scrolled tissue into the anterior chamber using unidirectional flow dynamics. Dr. Fogla connects the anterior chamber maintainer and uses a low flow rate prior to donor insertion. “This helps to keep the anterior chamber well-formed, which allows for easy insertion of the Geuder injector without engaging iris tissue,” he says. “Once the tip of the injector is in the anterior chamber, it’s rotated to ensure that the open end of the DM scroll within the injector is facing upwards, i.e., away from the iris.”
He then discontinues the flow in the anterior chamber maintainer and disconnects the IV tubing from it as well. “This allows the anterior chamber maintainer to leak fluid out of the anterior chamber,” he says. “The donor DM scroll is now gently injected into the anterior chamber. The insertion is relatively easy due to the absence of counter pressure from fluid build-up in the anterior chamber. This also helps to retain the configuration of the donor DM scroll, and there’s also reduced risk of spontaneous expulsion of the donor DM from the anterior chamber on withdrawing the injector tip.”
He says that once the double-scroll configuration is retained, it’s relatively easy to open the two edges of the scroll by surface tapping (Figure 1). “On average, it took approximately four minutes from donor insertion to complete unfolding of donor DM and securing it with an air bubble in our study,” he says. “More than 75 percent of eyes managed to achieve a visual acuity of 20/20 or better with an average endothelial cell loss of 16.7 percent at six months (versus 33 percent with conventional DMEK surgery).” Additionally, the two partial donor detachments (10 percent) that occurred in the study resolved spontaneously.
• Shark fin marking. Using a peripheral asymmetrical shark fin-shaped mark can help surgeons achieve correct intraoperative graft orientation, according to a study published last year.4 The technique, adapted from one proposed by surgeons at Moorfields Eye Hospital in 2015,5 is used at the end of the liquid-bubble preparation and consists of making a small excision on the graft margin with scissors.
The retrospective study of patients undergoing DMEK for Fuchs’ endothelial dystrophy included 184 patients receiving grafts without marks and 193 patients receiving grafts with a triangular mark. With the triangular mark, the authors reported significantly reduced postoperative graft turning rates (9 percent without a mark vs. 2 percent with a mark, p=0.002) and re-DMEK rates (8 percent without a mark vs. 2 percent with a mark, p=0.01). Re-DMEK due to primary graft failure was significantly associated with prior graft turning (p<0.001). The two groups’ postoperative outcomes were comparable for visual acuity, central corneal thickness and endothelial cell counts at three months. (The authors noted that this method is cost-effective.)
• Orienting the graft using a “bubble-tap” technique. The bubble-tap technique, first described by Georgios Perdikakis, MD, of the Clinic of Ophthalmology at St. Johannes Hospital in Dortmund, Germany, is an alternative DMEK orientation method that doesn’t involve ink marking, trephination of the graft or OCT-guided implantation. Recently, Dr. Perdikakis and his colleagues published their findings with this method in 500 eyes of 422 patients with Fuchs’ endothelial dystrophy, endothelial graft rejection following PK, bullous keratopathy following cataract surgery, and glaucoma shunt and MIGS implantation with DMEK.6
There was a significant improvement from mean preoperative to 12-month BCVA (0.39 ±0.49 vs. 0.76 ±0.26 decimal, or approximately 20/50 vs. 20/26 Snellen; p<0.001). There were no instances of primary graft failure due to upside-down graft implantation. Rebubbling was required in 4.8 percent of eyes, and re-DMEK was performed in 1.8 percent of cases due to graft failure. The authors say this technique is suitable for complicated cases with poor anterior chamber visibility.
After graft insertion into the anterior chamber, endothelium-down, and partial unfolding with no-touch tapping and BSS, a small 1- to 3-mm-diameter air bubble is injected underneath the graft. This bubble must remain under the graft at all times; it partially lifts the graft and supports it against the posterior stroma surface. Next, BSS is injected through a side port to deepen the anterior chamber, and the remaining unfolded graft edges are unfolded over the bubble using no-touch tapping.
Dr. Perdikakis says the most important part of this technique is to observe the graft edges that aren’t supported by the bubble when the peripheral corneal surface outside the bubble’s edges is tapped gently with the irrigating cannula. Peripheral tapping is repeated in several areas for 360 degrees. He says there will be little to no movement in the graft edges if it’s inserted correctly because the graft will have attached securely to the posterior corneal stroma. Grafts from older donors may exhibit slight movement due to laxity, however, so tapping around the entire graft is recommended. If the graft is upside down, the edges will roll toward the iris.
Unscrolling Challenges
The length of time and amount of manipulation it takes to unscroll a DMEK graft may affect the final outcome.7 Here are some techniques for graft unscrolling in difficult circumstances:
• When the endothelium-in graft doesn’t unscroll spontaneously.
Gillian Siu, MD, of the Department of Ophthalmology and Visual Sciences at Caritas Medical Centre in Hong Kong and her colleagues recently described a novel approach called the “side press-and-release technique” for situations in which the endothelium-in graft doesn’t unfold spontaneously.8 “If the anterior chamber isn’t sufficiently deep, or if the graft radius is large relative to the anterior chamber depth, the iris may prevent the graft from unfolding,” she explains.
“An endothelium-in graft behaves differently from an endothelium-out graft, so the traditional strategies for dealing with a scroll, double-scroll, taco, origami, etc., such as the no-touch technique, Dirisamer technique or Dapena maneuver,9-11 don’t apply here,” she says. “The side press-and-release technique is simple and safe; it works because it deals with the culprit that halts graft unfolding—the iris. As the name implies, it must be done at the periphery of the graft rather than the center, as with traditional DMEK.”
To perform the technique, the graft is tri-folded and loaded into a DMEK EndoGlide (Coronet Medical Technologies). It’s then pulled into the eye with a 27-gauge curved forceps under continuous anterior chamber infusion. The EndoGlide is removed from the main wound.
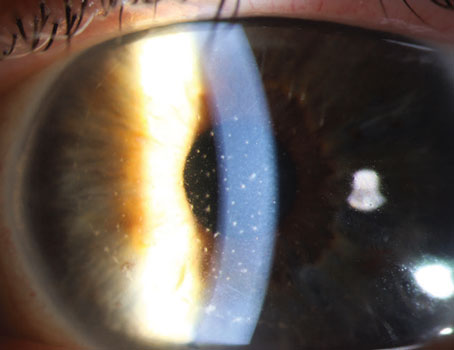
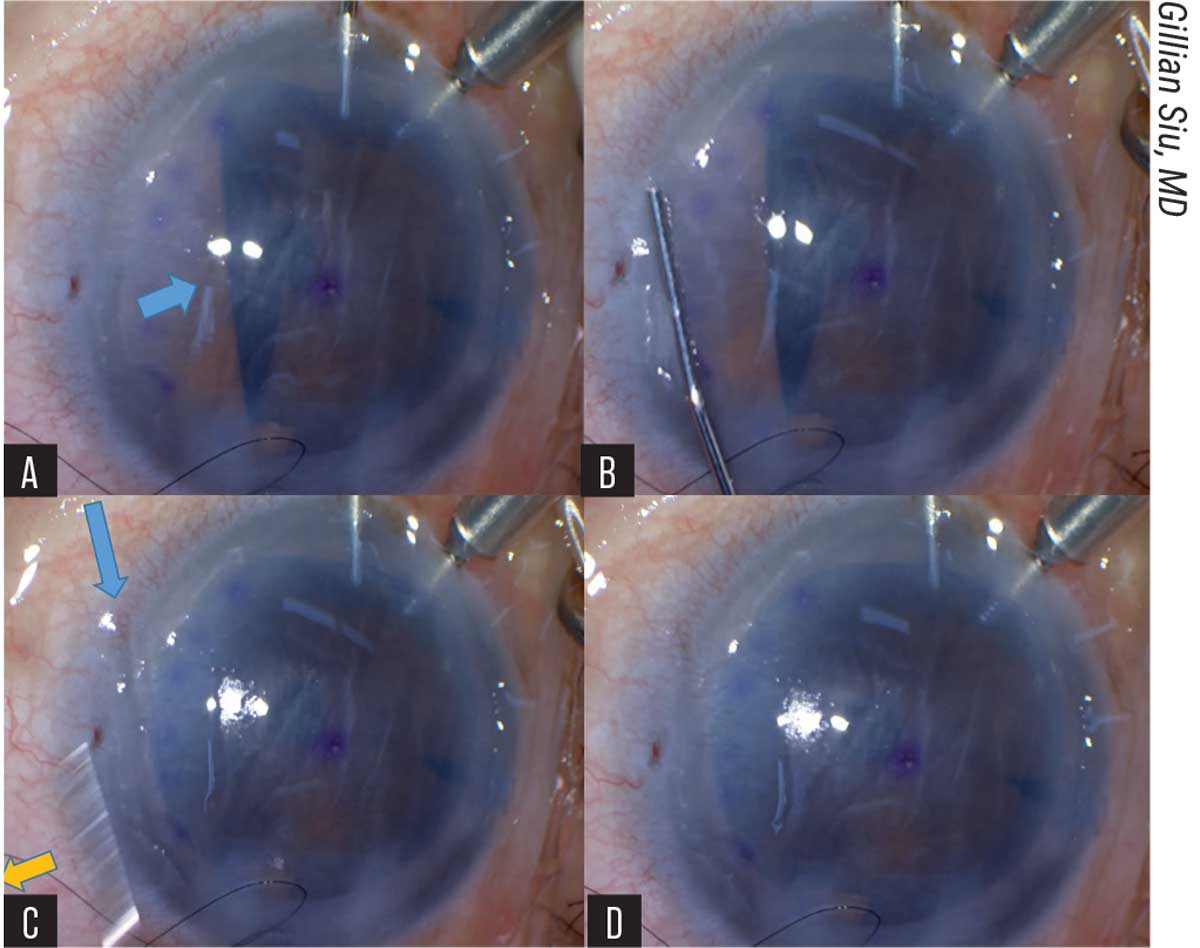 |
| Figure 2. (A) The blue arrow indicates the area of the unfolding graft caught by the iris. (B) A 27G cannula is pressed onto the peripheral cornea parallel to the axis of the folded graft. (C) The yellow arrow shows the swift release of the cannula from the cornea. The blue arrow shows the imprint of the cannula left on the cornea. (D) The side-press-and-release technique can be repeated until the graft unfolds completely. |
At this point, the pulled-through, endothelium-in DM graft resembles a bird ready to spread its wings, Dr. Siu says. The long axis is grasped with forceps and held still while the surgeon uses a 27-gauge cannula, placed parallel to the graft’s long axis, to press on the mid-peripheral cornea, directed towards the iris surface. The water current generated by the pressing pushes the iris diaphragm down, which frees the edge of the graft. As soon as the surgeon sees the freed graft edge, the cannula is released from the cornea. Dr. Siu says the sudden release from the cornea creates a suction force that unfolds the graft edge. This is repeated for any other folded graft edges caught by the iris (Figure 2).
She and her colleagues reported five successful cases using this technique in Chinese patients with bullous keratopathy. She offers the following tips for success:
— Ensure that the water current from the AC maintainer isn’t pressing onto the wings of the unfolding graft.
— Check that the graft isn’t trapped in either the main wound or the paracentesis that’s used to pull-through the graft.
— Don’t over-infuse the eye after graft insertion in an attempt to deepen the chamber and free the folded edges. There’s a potential risk of flushing the graft out of the eye.
— Don’t let go of the graft until a small gas bubble can be injected underneath. “Once the graft is let loose and scrolls up in its natural configuration,” she cautions, “even the traditional DMEK unfolding strategies may not be of much help, due to the folds that were created by folding the graft endothelium-in prior to insertion.”
• A no-touch maneuver for tightly scrolled grafts. “The spinning technique enables the surgeon to unfold tightly scrolled DMEK grafts without direct manipulation,” says Alfonso Vasquez-Perez, MD, FRCOphth, FEBOS-CR, of Moorfields Eye Hospital in London. “This minimizes the risk of endothelial cell loss. It also opens up the possibility of using younger donor tissue for DMEK, which has higher endothelial cell counts.”
He says that being able to use younger donor tissue more easily for DMEK will also help to address the tissue shortage. “Traditionally, only corneas from donors older than 50 years are selected for DMEK by most eye banks,” he notes. “We must remember that DMEK is now the most common procedure in many countries and its popularity is increasing. At Moorfields Eye Hospital in the U.K., currently half of the corneal transplants are DMEK. We’re now considering using donors from people in their 40s to avoid shortage issues.”
Dr. Vasquez-Perez says the DMEK spinning technique can be used for any case, but it’s particularly advantageous in eyes with very deep anterior chambers such as high myopes, vitrectomized eyes or buphthalmos, as opening a tightly scrolled DMEK graft in these cases is extremely challenging using classic cornea tapping maneuvers.12
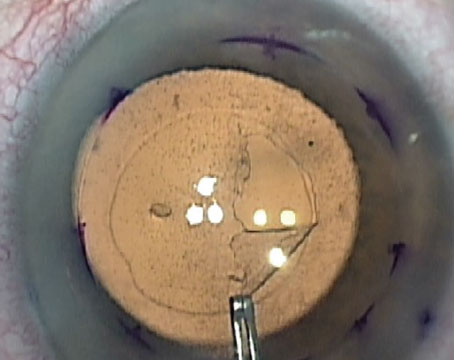
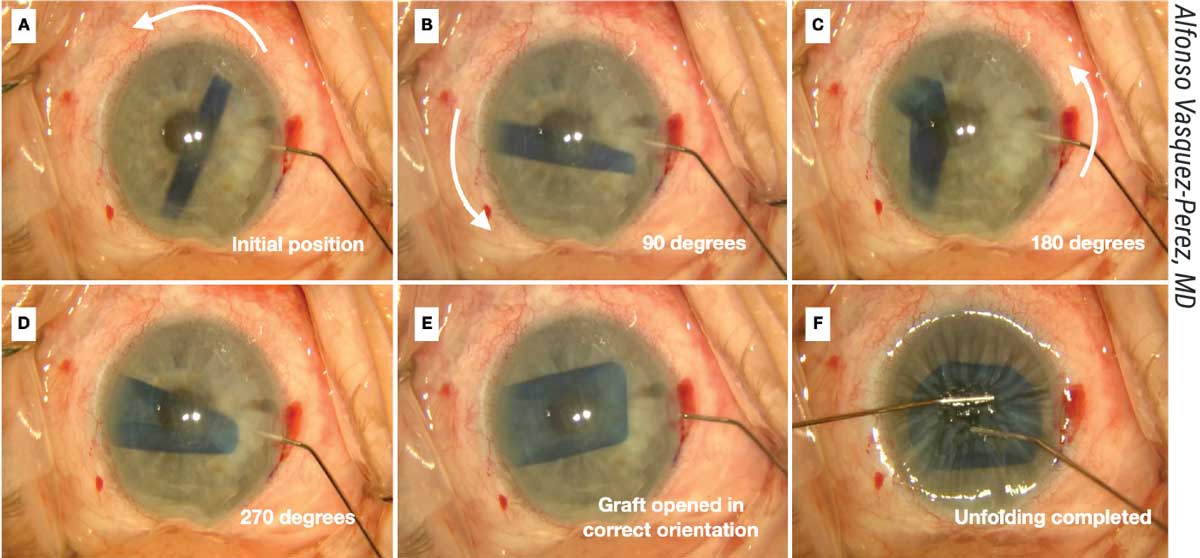 |
| Figure 3. (A) The spinning technique is useful for working with tightly scrolled grafts. (B-D Here, a 30-gauge Rycroft cannula is inserted through the paracentesis and short jets of BSS are applied to the distal side of the roll to create a 270-degree spin of the DMEK graft. (E-F) Unfolding is completed. |
He describes the basic steps for the technique (Figure 3):
“First, move the DMEK roll into a central position but perpendicular to one of the incisions,” he says. “The anterior chamber needs to be only partially filled. Next, a 30-gauge cannula with BSS is introduced through the incision with its tip pointing to one edge of the DMEK roll. Short jets of BSS are injected to make the graft spin.
“While it’s spinning over the iris, the graft rotates on its axis and unrolls as a result of centrifugal force,” he continues. “It usually opens in the correct orientation in a double scroll configuration. Once the graft has opened, immediate decompression of the anterior chamber must be done, followed by pressing the cornea with a cannula to prevent re-scrolling of the graft. The graft can then be opened completely using standard non-touch maneuvers followed by air injection to attach it to the posterior cornea.”
Dr. Vasquez-Perez recommends a 7.5- to 8-mm graft diameter. “Undersizing the graft will allow for enough space in the anterior chamber for spinning,” he says. “Additionally, be sure to alternate BSS injection with anterior chamber decompression during the spinning maneuver to prevent overfilling of the anterior chamber and graft re-scrolling.”
• Infusion forceps with the pull-through technique. Divya Srikumaran, MD, an associate professor of ophthalmology and Vice Chair of Education at the Wilmer Eye Institute, says her team’s pull-through technique using novel infusion forceps is especially useful for surgeons transitioning from DSAEK to DMEK and for cases where shallowing the anterior chamber is a challenge.13
Invented by her colleague Albert S. Jun, MD, PhD, chief of the cornea, cataract and external eye disease division at the Wilmer Eye Institute, the curved and extra-curved infusion microforceps can be used with a 1.1-mm paracentesis, Dr. Srikumaran says (Figure 4). “They have an irrigation sleeve that’s connected to a BSS-filled syringe,” she explains. “I usually use a 10-cc syringe with BSS to help irrigate and maintain the chamber.
 |
| Figure 4. Infusion forceps enable the surgeon to control chamber depth while pulling tissue into the eye. |
“The forceps have both a grasping function and an irrigation function, so you can pull tissue into the eye and control the chamber depth at the same time,” she explains. “In addition, you don’t need to have the chamber completely shallow in order to open the graft. This is especially useful when unscrolling tissue in long eyes with deep anterior chambers or during combined cataract and DMEK cases, where shallowing the chamber during the traditional DMEK dance technique could inadvertently shift the lens implants.”
When learning this technique, she says it’s helpful to have an experienced assistant or scrub tech to assist with irrigation while you execute the pull-through. “Once you’ve pulled the tissue into the eye, you have control over the infusion as well as the forceps, so you can ensure the tissue is centered before you release it into the eye,” she notes. “It’s also important to be familiar with the conventional tapping techniques to fall back on in case the graft isn’t unfolding as expected.”
She says this approach is well-suited for DMEK newcomers. “You can use DSAEK tissue for this technique,” she says. “DSAEK tissue is slightly thicker, so surgeons may feel more comfortable maneuvering it than the more fragile DMEK tissue. When starting out with any DMEK technique, and with this pull-through technique as well, choose straightforward cases. Don’t jump into a complex eye from the beginning.
“Using preloaded tissue also makes this technique easier,” she says. “Before the eye banks offered preloaded tissue, we’d trephinate and trifold the pre-stripped tissue, endothelium-in, with a little viscoelastic on the inner lining of the endothelial surface. Then we’d load it onto the spoon and draw it into the eye with forceps. Now, Lions VisionGift offers the tissue preloaded in a DMEK EndoGlide.”
Dr. Srikumaran uses the infusion forceps and the pull-through technique for both her DMEK and DSAEK cases. “I’ve used it in eyes with varying anterior chamber depths, but I haven’t been performing DMEK in unicameral eyes that have sutured lenses or eyes with more complex anterior segment anatomy since there’s a greater risk of graft detachments and other complications. I usually still offer DSAEK in those instances because it’s common for those patients to also have other comorbidities that will limit their ability to see after the surgery. However, we believe that this technique does hold promise for those complex eyes.”
She reports positive six-month findings in her case series (n=33), which assessed DMEK outcomes using the novel infusion forceps. Endothelial cell loss was 29.1 percent at six months for 20 eyes, and 82 percent achieved BCVA of 20/25 or better. Six eyes required rebubbling for graft detachments; there were no primary graft failures.
“This was our early experience using these forceps,” she says. “We didn’t have any significant complications, and graft detachment rates were similar to those in existing literature, as were the initial cell counts. The results seem promising. Many of these cases were performed by fellows who haven’t really done DMEK or DSAEK before their training, so we felt that this was a nice technique for beginning surgeons as well. We’re currently reviewing longer-term results with a larger number of eyes.”
DMEK in Complex Eyes
DMEK innovation is evolving to include approaches tailored to the unique challenges presented by complex eyes. Here are two new ones:
• Three-quarter DMEK. The three-quarter DMEK technique uses a modified 11- to 12-mm mega-graft placed centrally with the missing quarter positioned at the tube shunt location.14 Researchers at the NIIOS say the mega-graft may compensate for the missing tissue quarter in terms of absolute cell numbers.
Their case series of four eyes of three patients with pseudophakic bullous keratopathy after insertion of a glaucoma drainage device had clear corneas at the last follow-up and experienced no complications. Average central endothelial cell density was 1,093 ±74 cells/mm2 at 12 months postoperatively, which corresponded to an ECD decrease of 58 percent, compared to preoperative values. Patients’ average BCVA increased from count-fingers to 20/60 at 12 months and remained stable up to 24 months.
• For eyes with concomitant aphakia and aniridia. “Performing DMEK in aphakic-and-aniridic eyes was previously considered infeasible,” says Dr. Vasquez-Perez. “The main challenge was the lack of a surface to unfold the graft on. Additionally, without an iris or lens, there’s the risk of the graft luxating into the vitreous cavity. From our experience, however, unlike with DSAEK, luxated DMEK grafts don’t induce retinal detachments in vitrectomized eyes.”
Dr. Vasquez-Perez’ DMEK safe net technique for aphakia-and-aniridia allows these patients to benefit from DMEK, which has a significantly lower rejection rate compared to DSAEK traditionally performed in such eyes.15 He describes the steps:
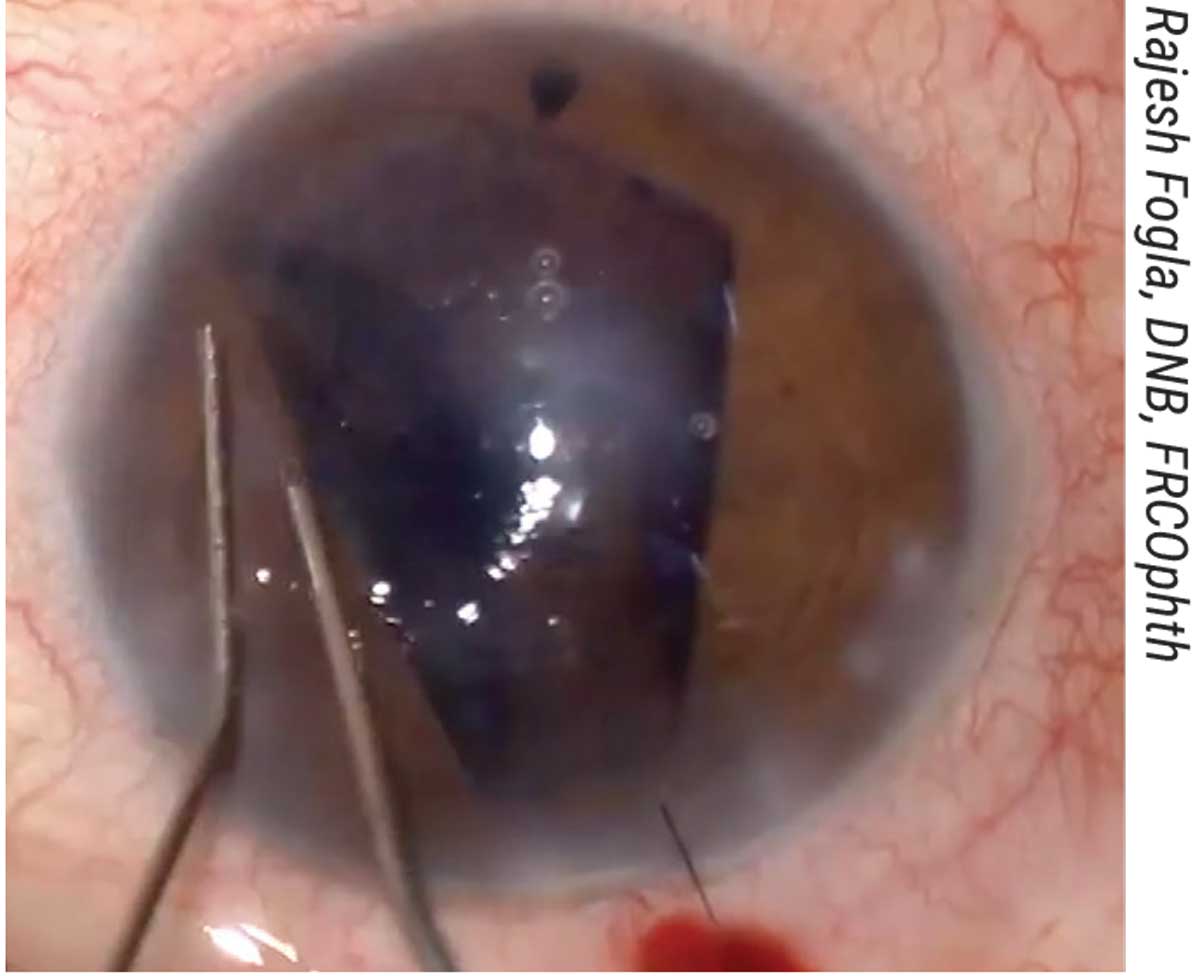
“We pass a 10-0 prolene straight needle suture at the level of the limbus multiple times (three in the horizontal plane and five in the vertical plane) to create a ‘net’ or scaffold over which the graft will be unfolded (Figure 5).
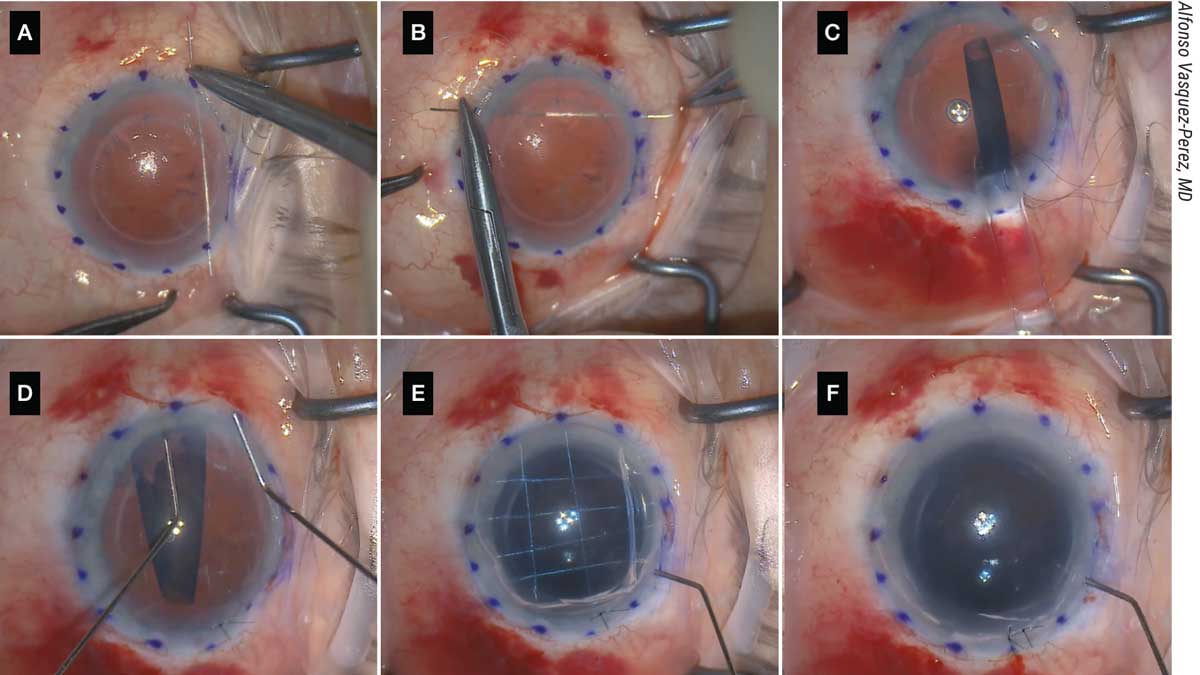 |
| Figure 5. (A, B) The patient shown here with aphakia and aniridia and previously failed DSAEK underwent the DMEK “safe net” technique, which involves continuously passing a 10-0 prolene suture horizontally and vertically to create a safety net. (C) The graft is then injected over the safety net; this must be done slowly and in a controlled manner. (D) Then, the graft is unfolded with two cannulas over the net in a shallow anterior chamber. (E) It’s attached to the cornea with air, and the safety net is visualized. (F) The prolene suture is removed and the DMEK graft remains attached. |
“Following this step, the corneal incision and paracentesis are created as usual, and descemetorrhexis is performed under air,” he continues. “The graft injection is the most important part: We use a 3-mm Geuder glass injector. The graft is loaded into the injector and moved carefully in a position near the tip to confirm the correct position for unfolding. With a Simcoe cannula, irrigation is maintained in the eye at the same time the injector is introduced and then rotated to allow injecting of the graft in the correct position for unfolding.
“With gentle tapping on the 3-ml syringe embolus attached to the injector, the graft is slowly injected over the safety net in a very controlled manner,” he says. “A corneal suture is placed to provide more stability, and graft unfolding is completed with standard bimanual tapping over the cornea. Air is then injected to attach the graft to the back surface of the cornea, and the prolene safe net suture is removed by cutting the edges and pulling the strings. Final tamponade is performed to pressurize the eye, leaving a 90-percent air volume in the anterior chamber.”
Dr. Vasquez-Perez says to succeed with this technique, the prolene safe net suture must be done before opening the eye. “Otherwise,” he says, “the aphakic-and-aniridic eye that had previous vitrectomy will suffer deformation and collapse when passing the sutures in the presence of open corneal incisions. I use a marking pen to mark the points where the needle needs to be directed. Good experience in performing DMEK is necessary for these cases. Mastering the injection and unfolding of the graft is essential.”
He adds that the detachment rates with this technique are similar to those of the standard technique in normal eyes. “Rebubbling can also be done at the slit lamp,” he notes. “Of the 10 cases performed with this new technique, only one case required a redo DMEK following rejection and failure. Usually these eyes have more complex pathology and higher rejection is expected. This is one of the reasons we prefer to do DMEK for cases that have the lowest immunological rejection.”
Preparing to Conserve Tissue
Developing techniques that increase tissue utilization will be needed to address acute shortages, according to experts. It’s estimated globally that for every 70 eyes, there’s only one corneal graft available,16 and the pandemic has further reduced the donor pool.
“The COVID-19 pandemic has affected all areas of medicine, including eye banking,” says Samar K. Basak, MD, FRCS, of Disha Eye Hospitals, Kolkata, India. “Stricter donor criteria have reduced the overall donor pool by 40 to 70 percent at various eye banks, including those in India; however, the demand for corneal tissue remains the same.”
Here are some techniques that may help to increase the number of potential recipients per donor cornea:
• A graft-marking method using a bandage contact lens. “The split-cornea concept of using the same donor for DALK and DMEK is one potential avenue,”17 Dr. Basak notes. “However, many surgeons aren’t comfortable with non-stamped DMEK-grafts since there’s the possibility of reversed unfolding, which may not be detected in the presence of a hazy cornea and in eyes with dark irises. That’s why most surgeons prefer tissues with an ‘S-mark’ or ‘F-mark’ on the DM-side for correct graft orientation. These are made through a 3-mm punched-out stromal window. Naturally, we can’t use this anterior tissue as a donor tissue for DALK or other surgeries.”
Instead, Dr. Basak and his colleagues recently proposed a novel bandage contact lens-assisted DMEK graft-marking technique that doesn’t require making a stromal window and preserves the anterior corneal tissue.18 “We use the BCL-interface technique for donor graft preparation, depending upon the immediate need for anterior corneal lamella on the same day,” he says.
With this approach, the intact anterior corneal lamella can be used on the same day for other types of keratoplasty. “This approach has helped during the worst of the pandemic when tissue availability was low,” Dr. Basak says. “In 2021, we used this BCL-interface technique in more than 80 eyes. Half of the anterior lamellar tissue was used for DALK. In other cases, we used this tissue for tectonic/large therapeutic grafts in globe-saving procedures and as carriers for Boston KPro.”
To prepare the graft using Dr. Basak’s method, the corneal button is first placed endothelial-side up on a Teflon block, and a 9.5-mm trephination is done. Trypan blue is applied to stain the cut margin of the DM-endothelial layer. The peripheral DM-E layer beyond the trephination mark is stripped off, and then a 9.5-mm DM-E layer is peeled off, starting from the distal peripheral cornea, with the help of McPherson’s forceps to keep a 1.5- to 2-mm hinge at the opposite end.
Next, a 14-mm bandage contact lens is placed on a second Teflon block, concave-side up, and resized with a 12-mm trephine. “This resizing is important so that the BCL is able to trap air while flipped and maintain a dome shape without collapsing,” Dr. Basak notes. “We use a central or paracentral 3-mm additional trephination of the BCL to make a window. The BCL is stained with trypan blue for better visibility, and then it’s placed carefully on the bare donor stroma with the concave-side up.
“The peeled off DM-E layer is then floated back over the BCL,” he continues. “The hinged (remaining) part of the DM-E layer is peeled off gently and the freed DM-E is centered over the BCL. Now, the BCL with the graft is flipped and air is trapped nicely, forming a dome with the DM-side facing up. S-stamping is done on the DM-side through a 3-mm window of BCL. Then the whole BCL with the graft is flipped back, concave-side up (endothelial-side up), and transferred to the second Teflon block.
“The final 7.5- to 8-mm trephination of the graft with the BCL is done with care,” he says. “With the help of McPherson’s forceps, the donor graft is shifted onto the bare donor stroma, which is on the first Teflon block. It’s stained with trypan blue for one minute and then washed with BSS. The DM scroll forms spontaneously due to its elastic properties, with the endothelial layer on the outer side.
“We transfer the stained and stamped DMEK graft to a petri dish containing BSS, so it’s ready for loading into an injector system made from an IOL cartridge (Figure 6),” he says. “The anterior corneal lamella is preserved temporarily in the same storage solution for subsequent use within the next two to three hours. As the first light punch was given with a 9.5-mm trephine, harvesting up to a 9.5-mm DALK graft isn’t a problem.”
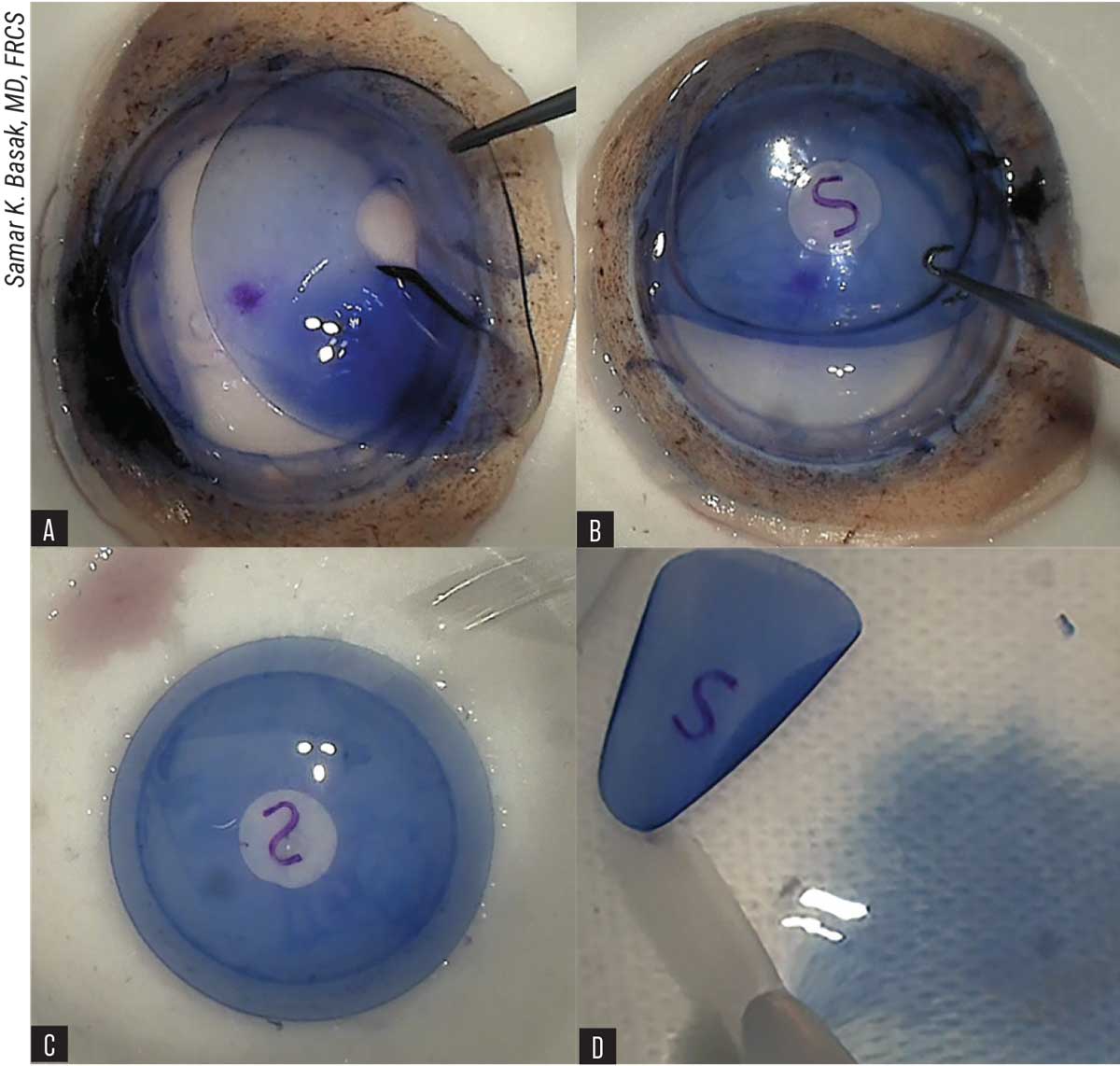 |
| Figure 6. BCL-interface technique of DMEK graft preparation. (A) Placement of resized bandage contact lens with central hole on the corneal stroma. (B) S-stamping on Descemet’s membrane-side via BCL window. (C) Reverse flipping of BCL with Descemet’s membrane-endothelium layer on the second Teflon block with endothelial side up. (D) Stained and stamped DMEK-graft is transferred to a petri dish, containing BSS and ready for loading. |
Here are some tips for success:
— Perform peripheral 360-degree loosening of the DM edge with a fine-tipped instrument after 9.5-mm light trephination.
— After placing the BCL between the stroma and DM, and positioning the DM-E over the BCL, dry the DM surface with a Weck-Cel sponge.
• Marking using a viscoelastic device. This technique, described by Adi Einan-Lifshitz, MD, and colleagues, enables a single donor cornea to be used for DMEK while preserving the anterior cornea for another surgery using the stroma and Bowman’s layer.19
Their technique involves placing Healon5 (Johnson & Johnson Vision), a viscoadaptive OVD, over the endothelial side of DM. The DM is folded in half over the OVD, stromal-side up, and marked with an F on the stromal side. The researchers say that drying the stromal side before marking is key. The folded DM is then replaced onto the stromal bed, and the OVD is irrigated off the donor cornea.
The researchers say they chose Healon5 because it behaves as a super-viscous cohesive device when exposed to low BSS flow rates, and because it can be gently washed away from the DMEK graft. In their retrospective case analysis of 26 eyes, mean BSCVA improved from 1 ±0.7 logMAR (20/200) at baseline to 0.4 ±0.4 logMAR (20/50) at 12 months postop. Primary failure occurred in 11 percent of eyes, and 27 percent of eyes had partial graft detachment requiring air injection. The authors say this partial graft detachment and rebubble rate is comparable to the AAO’s reported 28.8 percent.20 No free-floating donors or inverted donors occurred, and the endothelial cell loss rate was 38.3 percent at 12 months.
• Customized DMEK. DMEK is rising in popularity, as is the demand for high-quality endothelial grafts, according to Isabel Dapena, MD, PhD, of the NIIOS in her 2021 Subspecialty Day presentation at the American Academy of Ophthalmology meeting in New Orleans. She explained that while quarter- and hemi-DMEK conserve tissue and clear the cornea faster than Descemet’s Stripping Only,21,22 endothelial cell density remains lower than with standard DMEK, likely due to the migration of endothelial cells covering the stromal gaps surrounding the graft. She noted that these approaches may also have a higher tendency to detach than circular grafts.
Researchers at the NIIOS proposed a customized DMEK approach, which tailors the size of the descemetorhexis and circular graft to the extent of an individual patient’s corneal damage.23 She explained in her presentation that using a smaller graft to replace only the affected area of endothelium could potentially better preserve peripheral host cells and offer visual recovery comparable to standard DMEK with a lower risk of graft rejection.
None of the doctors interviewed for this article report relevant financial disclosures.
1. Tran KD, Dye PK, Odell K, et al. Evaluation and quality assessment of prestripped, preloaded Descemet membrane endothelial keratoplasty grafts. Cornea 2017;36:4:484–490.
2. Wolle MA, DeMill DL, Johnson L, et al. Quantitative analysis of endothelial cell loss in preloaded descemet membrane endothelial keratoplasty grafts. Cornea 2017;36:11:1295-1301.
3. Fogla R, Thazethaeveetil IR. A novel technique for donor insertion and unfolding in Descemet membrane endothelial keratoplasty. Cornea 2021;40:8:1073-1078.
4. Rickmann A, Boden K, Trouvain AM, et al. Clinical results after single asymmetrical shark fin for graft orientation in DMEK. Int Ophthalmol 2021. [Epub ahead of print].
5. Bhogal M, Maurino V, Allan BD. Use of a single peripheral triangular mark to ensure correct graft orientation in Descemet membrane endothelial keratoplasty. J Cataract Refract Surg 2015;41:9:2022-2024.
6. Perdikakis G, Fili S, Vastardis I, et al. The correct graft orientation during Descemet membrane endothelial keratoplasty (DMEK) using the “bubble-tap” technique. Int Ophthalmol 2021;41:7:2329-2337.
7. Baur A, et al. The relationship between DMEK graft scroll tightness and clinical outcomes. IOVS 2020;61:7:3565. Presented at the 2020 ARVO Annual Meeting.
8. Siu GD, Wu MM, Wong AL. Side press-and-release technique in endothelium-in Descemet membrane endothelial keratoplasty (DMEK): A novel technique. Eur J Ophthalmol 2021. [Epub ahead of print, October 16, 2021].
9. Liarakos VS, Dapena I, Ham L, et al. Intraocular graft unfolding techniques in descemet membrane endothelial keratoplasty. JAMA Ophthalmol 2013; 131: 29–35.
10. Liu C, Vasquez-Perez A, Chervenkoff JV, et al. Vent incisions to facilitate peripheral unfolding of the DMEK graft. Cornea 2017; 36: 1150–1154.
11. Sarnicola C, et al. Cannula-assisted technique to unfold grafts in descemet membrane endothelial keratoplasty. Cornea 2019; 38: 275–279.
12. Vasquez-Perez A, Phylactou M, Din N, et al. “The Spinning Technique” for unfolding tightly scrolled DMEK grafts. Cornea 2022;41:1:130-134.
13. Jabbour S, Jun AS, Shekhawat NS, et al. Descemet membrane endothelial keratoplasty using a pull-through technique with novel infusion forceps. Cornea 2021; 40:3:387-392.
14. Oganesyan O, Makarov P, Grdikanyan A, et al. Three-quarter DMEK in eyes with glaucoma draining devices to avoid secondary graft failure. Acta Ophthalmol 2020. [Epub ahead of print].
15. Vasquez-Perez A, et al. DMEK safe net technique for aphakia&aniridia. Cornea 2022. In Press.
16. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016;143:2:167-73.
17. Gadhvi KA, et al. Eye banking: One cornea for multiple recipients. Cornea 2020;39:1599-1603.
18. Basak SK, Basak S. Marking DMEK grafts using bandage contact lens interface technique: Doubling the utilization during the acute shortage of donor corneas. Cornea 2021. [Epub ahead of print November 11, 2021].
19. Or L, et al. A novel marking technique for Descemet membrane endothelial graft using an ophthalmic viscoelastic device. Cornea 2021;40:4:529-532.
20. Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology 2018;125:295-310.
21. Birbal RS, Ni Dhubhghaill S, Baydoun L, et al. Quarter-Descemet membrane endothelial keratoplasty: One- to two-year clinical outcomes. Cornea 2020;39:277-282.
22. Birbal RS, Hsien S, Zygoura V, et al. Outcomes of hemi-Descemet membrane endothelial keratoplasty for Fuchs endothelial corneal dystrophy. Cornea 2018;37:854-858.
23. Dapena I, Melles G, et al. New DMEK Techniques. Presented at Cornea Subspecialty Day at the American Academy of Ophthalmology meeting in New Orleans, 2021.