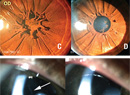
Cortical/cerebral visual impairment is the leading cause of pediatric visual impairment in the United States and other developed economies.1 CVI can be challenging to diagnose. In infants with visual impairment, the differential includes inherited retinal disorders, oculomotor apraxia, and delayed visual maturation. In older children, CVI can be confused with autism spectrum disorder or learning disabilities. Here, we’ll break down the diagnostic cues and tests that can help you make a definitive CVI diagnosis in a young patient.
 |
|
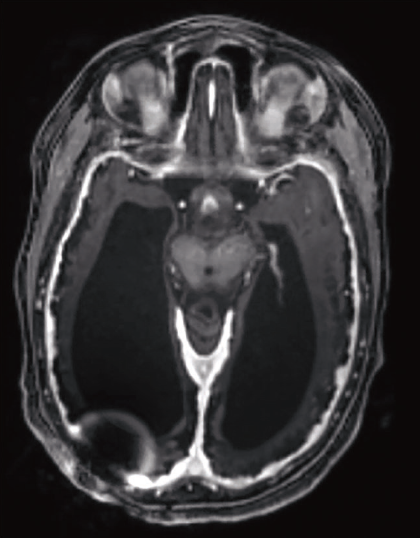
Figure 1. Brain magnetic resonance imaging scan of a 5-year-old child with cortical/cerebral visual impairment and multiple contributing etiologies, including hydrocephalus with a ventriculo-peritoneal shunt (artifact seen on the scan), hypoxic-ischemic encephalopathy with extensive cerebral volume loss, and seizures. |
CVI Background
CVI has been defined broadly as a “verifiable visual dysfunction which cannot be attributed to disorders of the anterior visual pathways or any potentially co-occurring ocular impairment.”2 A stricter definition of CVI requires “bilateral visual acuity or visual field loss in the presence of a normal eye examination or vision loss that is greater than expected based on the degree of ocular pathology.”1 Though the exact definition of CVI varies in the literature, most diagnostic criteria require: 1) a demonstrable abnormality of vision; 2) no ocular findings sufficient to explain the visual abnormality; and 3) a neurologic condition that affects the developing visual pathways in the brain.
Risk Factors for CVI
CVI is heterogeneous in etiology. However, there are common causes to be aware of. They include:
• prematurity with periventricular leukomalacia;
• term birth with perinatal hypoxic-ischemic encephalopathy;
• hydrocephalus;
• seizures (particularly those that are associated with epileptic encephalopathy, such as infantile spasms);
• trauma;
• infections, including meningitis and encephalitis;
• structural brain abnormalities (such as schizencephaly and colpocephaly);
• metabolic conditions such as hypoglycemia; and
• genetic disorders.1,3-6
In many cases, CVI is multifactorial and it may not be possible to determine which underlying neurologic condition is responsible for the visual dysfunction (Figure 1). Children with any of the aforementioned neurologic disorders should be considered at risk for CVI, and developmental pediatricians and neurologists may refer such patients for CVI evaluation.
Characteristics of CVI
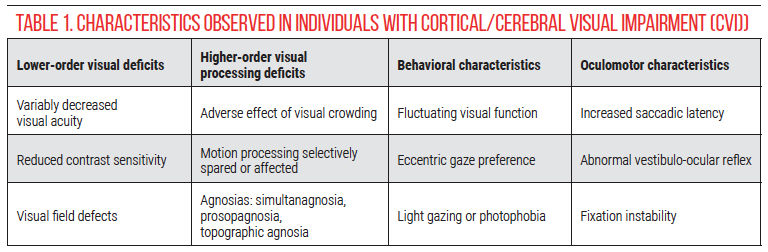
CVI is also heterogeneous in its visual manifestations (Table 1). Visual acuity may range from no light perception to age-normal, though the majority of patients evaluated by pediatric ophthalmologists have profoundly reduced visual acuity.7 Other characteristics include visual field defects, reduced contrast sensitivity, relatively spared color discrimination, and a global motion processing deficit or, paradoxically, relatively preserved motion perception.8-11 Visual behaviors associated with CVI include difficulty with visual search or using vision in crowded environments, highly variable visual function (visual behavior is often worse when the child is ill or fatigued), eccentric gaze preference (looking away while reaching), and light gazing or, paradoxically, photophobia.8,12,13 In children with good visual acuity, higher-order visual processing abnormalities may be detected, including difficulties with recognition, orientation, depth perception and simultaneous perception.14,15 Abnormal oculomotor behavior such as increased time to generate saccades when a visual stimulus is shown (latency), decreased or absent vestibulo-ocular reflexes, and fixation instability have also been described in CVI.16, 17
 |
Differential Diagnosis
The differential diagnosis of CVI depends on the age and presentation. In infants that are apparently blind, other diagnostic considerations include delayed visual maturation; oculomotor apraxia (prior to the development of neck control, which is required for head thrusting); and inherited retinal disorders. In older children with relatively good visual acuity, CVI may be confused with neurodevelopmental disorders such as autism spectrum disorder and learning disabilities, including dyslexia.
Evaluation and History
The evaluation of a child with suspected CVI should focus on elements that differentiate CVI from other conditions, and characterization of the severity of visual impairment in the affected child.
Structured history-taking questionnaires are available to elicit behaviors characteristic of CVI.18-21 These questionnaires are particularly helpful in children with good visual acuity, suspected higher-order visual deficits and limited communication.
In all children with suspected CVI, important historical questions include:
- Was the child born premature? Were there any birth complications? Was there a history of hypoxic-ischemic encephalopathy or cooling?
- Is there a family history of neurologic or ophthalmologic conditions?
- Was the child exposed to any drugs in utero?
- Does the child have any known neurologic conditions? If so, are they controlled (e.g., seizure activity)?
- Has the child undergone prior neuroimaging or genetic testing?
Examination
 |
A complete pediatric ophthalmologic examination is critical to support the diagnosis of CVI and rule out other entities in the differential diagnosis above. Important components of the examination include:
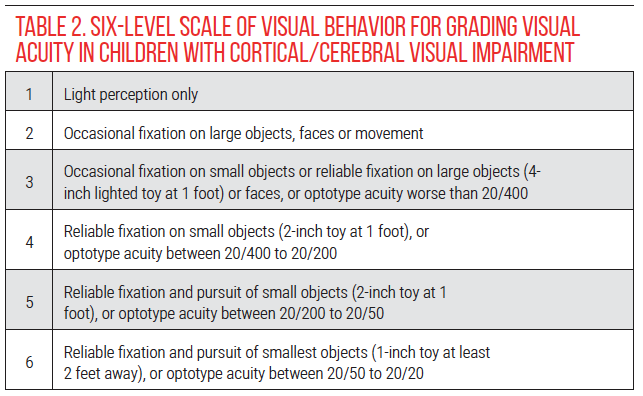
• Visual acuity. In children with CVI who are unable to cooperate with optotype acuity testing, a 6-level scale of visual behavior may be used to grade visual acuity and monitor changes over time (Table 2).4, 22
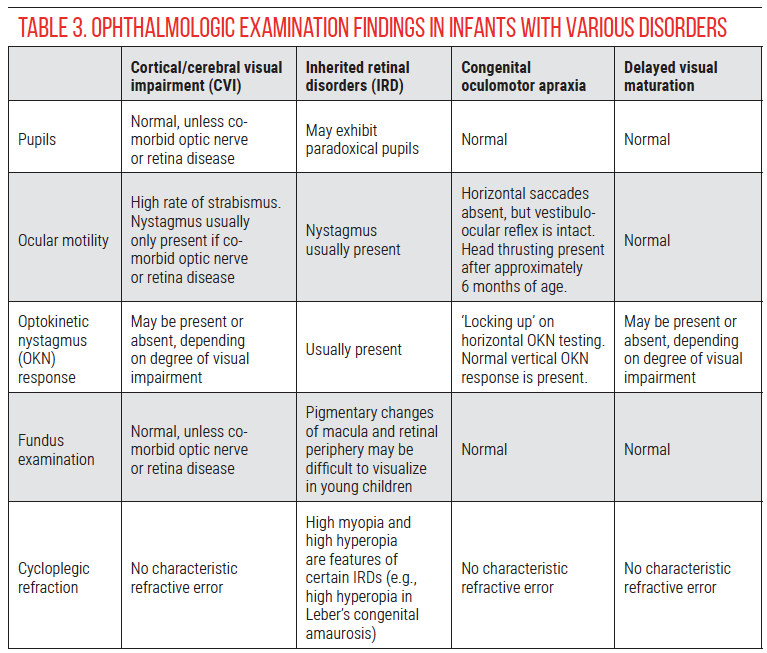
• Pupils. In addition to assessing pupillary reactivity and presence of an afferent pupillary defect, it’s important to evaluate the response to dark conditions in order to assess for paradoxical pupils. To check for paradoxical pupils in children, it’s best to have a second person control the light switch while the child’s attention is directed to a target in the distance (such as a toy or movie). The examiner uses a penlight for oblique illumination and observes the pupillary response when the light is turned off. In patients with paradoxical pupils, the pupils will initially constrict before dilating. The process may need to be repeated several times to observe a consistent response. Paradoxical pupils are a characteristic of inherited retinal disorders that may be mistaken for CVI.23
• Ocular motility. Children with CVI frequently have strabismus, which can change over time as their visual acuity improves.24,25 The presence of nystagmus should raise the suspicion for an inherited retinal disorder, since nystagmus isn’t typically a feature of CVI without anterior visual pathway dysfunction.
• Optokinetic nystagmus (OKN). In oculomotor apraxia, pursuit is intact whereas saccades are absent,26 resulting in ‘locking up’ (see referenced video27 for example). Check horizontal and vertical OKN response, as vertical saccades are usually spared in congenital oculomotor apraxia. Children with CVI may have absent OKN responses both horizontally and vertically, particularly when visual acuity is poor.
• Fundus examination. Subtle abnormalities suggestive of an inherited retinal disorder may be seen on careful fundus examination. Many children with CVI have optic nerve pathology, most commonly optic atrophy,1 but the vision loss must be greater than expected based on the optic nerve appearance in order to make a diagnosis of CVI.
• Cycloplegic refraction. High hyperopia or myopia may be a sign of an inherited retinal disorder,28 although this is non-specific. CVI is also associated with a high rate of refractive errors, nearly equally divided between myopia and hyperopia.29
Table 3 provides a summary of examination findings that distinguish between CVI and the primary differential diagnoses in infants with apparently poor vision: inherited retinal disorders, oculomotor apraxia and delayed visual maturation.
In children with suspected CVI who have relatively good visual acuity, abnormalities of higher-order visual processing may not be identified on standard pediatric ophthalmologic examination, and specialized testing by allied health professionals may be required.
 |
Diagnostic work-up
While there is no single test to diagnose CVI, the following ancillary tests/imaging may be necessary to identify underlying neurologic conditions, rule out differential diagnoses and characterize the severity of CVI.
• Ophthalmic electrophysiology. Electroretinography and/or visual evoked potentials may be indicated in some children with suspected CVI. ERG is most useful to evaluate for an inherited retinal disorder in young children with poor vision and nystagmus. Flash VEP may have limited prognostic value. Sweep VEP has been used to assess grating acuity,30 Vernier acuity,31 and contrast sensitivity10 in children with CVI, but obtaining reliable measurements in the most severely affected patients may be difficult or impossible. In general, electrophysiology isn’t required in most cases to make a diagnosis of CVI.
• Neuroimaging. If not previously performed, children with suspected CVI should undergo brain magnetic resonance imaging to assess for structural abnormalities of the posterior visual pathway.
However, some children with CVI (especially those with genetic disorders) have normal structural brain MRI scans. Newer MRI techniques, such as diffuse tensor imaging, have demonstrated better structure-function correlation in some studies, but these are generally accessible only in the research setting.32
• Genetic testing. Genetic disorders are increasingly recognized as a cause of CVI. Diverse conditions including seizure disorders, leukodystrophies, congenital disorders of glycosylation and many others have been associated with CVI.33 Genetic testing should be considered in children with CVI with syndromic features, especially when the etiology is unknown. Referral to medical genetics and a genetic counselor may be required to interpret the results. The American Board of Genetic Counseling maintains an online directory of certified genetic counselors at (https://abgc.learningbuilder.com/Search/Public/MemberRole/Verification).
• Functional vision assessments. Typically administered by teachers of the visually impaired or occupational therapists, tests of functional vision evaluate how a child uses his/her vision in everyday activities. This assessment is used to guide interventions, particularly in school, to allow the child to access educational material. The most widely used functional vision assessment is the CVI Range.34 However, other assessments are being developed and validated. More information on CVI assessments may be found on the Perkins School for the Blind’s “CVI Now” website (https://www.perkins.org/getting-started-with-cvi-assessments/).
• Neuropsychological assessments. Though the details are beyond the scope of this article and an ophthalmologist’s practice, neuropsychological assessments may be particularly helpful to diagnose CVI in children with suspected higher-order visual perceptual deficits in the setting of good visual acuity and to evaluate them for neurodevelopmental disorders that may be mistaken for CVI, including autism spectrum disorder (ASD) and dyslexia. It’s important to note that CVI may be co-morbid with these neurodevelopmental conditions, as vision is vital for both social interactions and reading text.35 However, in order to diagnose CVI in the context of other neurodevelopmental disorders, children must have deficits specific to CVI that aren’t explainable by the other diagnoses. Assessments for ASD and dyslexia are usually provided by state-sponsored early intervention resources and school districts, although some parents may seek private evaluations.
In summary, CVI is a common and heterogeneous disorder that impacts functional vision. Pediatric ophthalmologists have an important role in suspecting CVI, guiding further diagnostic work-up, and ultimately providing the diagnosis. Recommendations for evaluating children with suspected CVI include:
• Consider incorporating CVI-specific questionnaires to identify higher-order visual perceptual deficits that are difficult to elicit in clinic.
• Conduct a thorough examination, paying careful attention to visual acuity (using the 6-level scale of visual behavior if needed), pupillary response to light and dark (particularly noting any paradoxical responses), presence of nystagmus and OKN response both horizontally and vertically, fundus examination (looking for subtle abnormalities of the retina and optic nerve) and cycloplegic refraction.
• ERG and VEP are generally not required for a CVI diagnosis, but ERG may be helpful to rule out an inherited retinal disorder in select patients (e.g. infants with poor vision and nystagmus, positive family history, and/or absence of risk factors for CVI).
• Consider brain MRI if not previously performed.
• Consider genetic evaluation in patients with syndromic features and no known risk factor for CVI.
• Obtain a functional vision assessment by a licensed professional, such as a teacher for the visually impaired or occupational therapist with expertise in CVI.
• Refer for neuropsychological evaluation if neurodevelopmental disorders (such as autism spectrum disorder) or learning disabilities (such as dyslexia) are suspected.
Albert Yang is a medical student at the Keck School of Medicine, University of Southern California, Los Angeles.
Dr. Chang is an assistant professor of clinical ophthalmology at the Keck School of Medicine and an attending physician at the Vision Center at the Children’s Hospital Los Angeles.
Corresponding author:
Melinda Y. Chang, MD
4650 Sunset Blvd., Mailstop #88
Los Angeles, CA 90027
E-mail: Melinda.y.wu@gmail.com
Phone number: (323) 361-4510
1. Chang MY, Borchert MS. Advances in the evaluation and management of cortical/cerebral visual impairment in children. Surv Ophthalmol 2020;65:6:708-24.
2. Sakki HEA, Dale NJ, Sargent J, et al. Is there consensus in defining childhood cerebral visual impairment? A systematic review of terminology and definitions. Br J Ophthalmol 2018;102:4:424-32.
3. Good WV, Jan JE, DeSa L, et al. Cortical visual impairment in children. Surv Ophthalmol 1994;38:4:351-64.
4. Huo R, Burden SK, Hoyt CS, Good WV. Chronic cortical visual impairment in children: Aetiology, prognosis, and associated neurological deficits. Br J Ophthalmol 1999;83:6:670-5.
5. Khetpal V, Donahue SP. Cortical visual impairment: Etiology, associated findings, and prognosis in a tertiary care setting. J AAPOS 2007;11:3:235-9.
6. Whiting S, Jan JE, Wong PK, et al. Permanent cortical visual impairment in children. Dev Med Child Neurol 1985;27:6:730-9.
7. Jimenez-Gomez A, Fisher KS, Zhang KX, et al. Longitudinal neurological analysis of moderate and severe pediatric cerebral visual impairment. Front Hum Neurosci 2022;16:772353.
8. Jan JE, Groenveld M, Sykanda AM, Hoyt CS. Behavioural characteristics of children with permanent cortical visual impairment. Dev Med Child Neurol 1987;29:5:571-6.
9. Pamir Z, Bauer CM, Bailin ES, et al. Neural correlates associated with impaired global motion perception in cerebral visual impairment (CVI). Neuroimage Clin 2021;32:102821.
10. Good WV, Hou C, Norcia AM. Spatial contrast sensitivity vision loss in children with cortical visual impairment. Invest Ophthalmol Vis Sci 2012;53:12:7730-4.
11. Chandna A, Nichiporuk N, Nicholas S, et al. Motion processing deficits in children with cerebral visual impairment and good visual acuity. Invest Ophthalmol Vis Sci 2021;62:14:12.
12. Jan JE, Groenveld M, Anderson DP. Photophobia and cortical visual impairment. Dev Med Child Neurol 1993;35:6:473-7.
13. Manley CE, Bennett CR, Merabet LB. Assessing higher-order visual processing in cerebral visual impairment using naturalistic virtual-reality-based visual search tasks. Children (Basel) 2022;9:8.
14. Dutton G, Ballantyne J, Boyd G, et al. Cortical visual dysfunction in children: a clinical study. Eye (Lond) 1996;10 (Pt 3):302-9.
15. Bauer CM, Manley CE, Ravenscroft J, et al. Deficits in face recognition and consequent quality-of-life factors in individuals with cerebral visual impairment. Vision (Basel) 2023;7:1.
16. Mansukhani SA, Ho ML, Brodsky MC. Abnormal vestibular-ocular reflexes in children with cortical visual impairment. J Neuroophthalmol 2021;41:4:531-6.
17. Salati R, Borgatti R, Giammari G, Jacobson L. Oculomotor dysfunction in cerebral visual impairment following perinatal hypoxia. Dev Med Child Neurol 2002;44:8:542-50.
18. Dutton GN, Calvert J, Ibrahim H, et al. Structured clinical history-taking for cognitive and perceptual visual dysfunction and for profound visual disabilities due to damage to the brain in children. In: Clinics in developmental medicine. London: MacKeith Press, 2010.
19. Chandna A, Ghahghaei S, Foster S, Kumar R. Higher visual function deficits in children with cerebral visual impairment and good visual acuity. Frontiers in Human Neuroscience 2021;15:711873.
20. Ben Itzhak N, Vancleef K, Franki I, et al. Visuoperceptual profiles of children using the Flemish cerebral visual impairment questionnaire. Dev Med Child Neurol 2020;62:8:969-76.
21. Macintyre-Beon C, Young D, Calvert J, et al. Reliability of a question inventory for structured history taking in children with cerebral visual impairment. Eye (Lond) 2012;26:10:1393.
22. Chang MY, Borchert MS. Validity and reliability of eye tracking for visual acuity assessment in children with cortical visual impairment. J AAPOS 2021;25:6:334:e1- e5.
23. Khan AO. Phenotypes and genotypes underlying paradoxical pupillary reaction in children. J AAPOS 2022;26:4:205-7.
24. Binder NR, Kruglyakova J, Borchert MS. Strabismus in patients with cortical visual impairment: Outcomes of surgery and observations of spontaneous resolution. J AAPOS 2016;20:2:121-5.
25. Handa S, Saffari SE, Borchert M. Factors associated with lack of vision improvement in children with cortical visual impairment. J Neuroophthalmol 2018;38:4:429-33.
26. Chang MY, Grosrenaud P, Borchert MS. Characteristics and outcomes of idiopathic and non-idiopathic ocular motor apraxia in children. J Pediatr Ophthalmol Strabismus 2022;59:5:326-31.
27. Im DH, Borchert MS, Chang MY. Delayed diagnosis of childhood-onset Huntington disease in an 8-year-old boy with ocular motor apraxia. J Neuroophthalmol 2023;43:4:e304-e5.
28. Hendriks M, Verhoeven VJM, Buitendijk GHS, et al. Development of refractive errors-what can we learn from inherited retinal dystrophies? Am J Ophthalmol 2017;182:81-9.
29. Rice ML, Sandoval MA, Castleberry KM, Schwartz TL. Physician prescribing and referral patterns in children with cerebral visual impairment. Optom Vis Sci 2021;98:9:1078-84.
30. Good WV. Development of a quantitative method to measure vision in children with chronic cortical visual impairment. Trans Am Ophthalmol Soc 2001;99:253-69.
31. Skoczenski AM, Good WV. Vernier acuity is selectively affected in infants and children with cortical visual impairment. Dev Med Child Neurol 2004;46:8:526-32.
32. Bennett CR, Bauer CM, Bailin ES, Merabet LB. Neuroplasticity in cerebral visual impairment (CVI): Assessing functional vision and the neurophysiological correlates of dorsal stream dysfunction. Neurosci Biobehav Rev 2020;108:171-81.
33. Bosch DG, Boonstra FN, de Leeuw N, et al. Novel genetic causes for cerebral visual impairment. Eur J Hum Genet 2016;24:5:660-5.
34. Roman-Lantzy C. Cortical visual impairment: An approach to assessment and intervention. 2nd ed. Louisville, Kentucky: AFB Press, 2007.
35. Chokron S, Kovarski K, Dutton GN. Cortical visual impairments and learning disabilities. Front Hum Neurosci 2021;15:713316.