Joseph Capriotti, MD, Aron Shapiro and Lauren Lilyestrom
The burnt-orange color of the surgical scrub made with polyvinylpyrrolidone-iodine, or povidone-iodine (PVP-I) for short, is easily recognized by the average patient as an antimicrobial precaution. But even this common polymer complex harbors some secrets and intriguing chemical properties, all of which can influence its antiseptic potential. In this article, we'll delve into these hidden aspects of the compound and explain how they can be best put to use in the prevention of infection.
The Halogen Family
Within a periodic group (a.k.a. column, or family), all the elements share similar features of their outermost electron shell. The halogen family, of which iodine is a member, is distinguished from other elemental families in that its constituent elements have seven valence electrons, one short of a stable octet. As a result, they seek to acquire the final electron from the surrounding molecules, making them potent disruptors of biological activity. This intrinsic high electronegativity prevents halogens from existing in nature as single elements, and most can exist only as divalent molecular compounds, as components of organohalogen complexes or as ionic salts. Iodine's location at the bottom of the halogen column on the period table helps explain its reduced, yet potent, reactivity and increased biocompatibility, since the atomic radius, number of electron shells and the size of the electron cloud increase for each element as you travel down a family on the periodic table. Valence electrons in such elements reside farther away from nucleus, reducing the element's electronegativity and, therefore, its reactivity.
The antimicrobial abilities of the halogens are rooted in their reactivity. Fluorine, for example, is a highly corrosive and toxic gas but, when reduced, its usability expands. The cavity protection afforded by fluoride is the result of fluoride promoting the formation of harder tooth enamel as well as its disruption of bacterial metabolism.1 The next two halogens, chlorine and bromine, are routinely used as disinfectants and bleaching agents, albeit in minute quantities.
Iodine is the least reactive halogen, the most biologically compatible, and is essential for the production of thyroid hormones. Like all halogens, it's seldom found in nature unless compounded or ionized. PVP forms a polymeric complex with elemental iodine that improves tolerability, stability and solubility in water. Free iodine is delivered from the PVP-I complex where it is carried in a less-irritating form. It's this delivery of free iodine that provides for the rapid microbicidal activity of PVP-I. In-vitro analysis paradoxically suggests that solutions with a lower concentration may be more effective than those with higher concentrations. This behavior is likely explained by the ability of less-concentrated solutions to deliver a higher concentration of free iodine outside the PVP-I complex due to kinetic factors that govern the intrinsic coiling and hydration dynamics of the PVP polymer itself. Numerous studies have attempted to find the most effective concentration of PVP-I, as well as the best method of application, but are hindered by many variables and clinically vague endpoints. As biochemists continue to unravel the activity of PVP-I, we're left to decipher the most effective method to use this indispensable surgical tool.
Lugol's Solution
Although iodine the element wasn't isolated until the 19th century, the antimicrobial properties of iodine-containing substances, particularly seaweed, were well-known and utilized long before: Aristotle's pupil Theophrastus described the pain relief provided by seaweed for wounds from sunburns.2 Iodine was first isolated in
His aqueous solution, Strong Iodine Solution USP XXIII, is still used today for such varied applications as cell staining, antisepsis and as an aquarium supplement.
As a bactericidal agent, iodine penetrates bacterial cell walls, and although its precise killing mechanism is uncertain due to its extensive halogen reactivity, it's likely related to retardation of bacterial protein synthesis, disruption of electron transport, DNA denaturation or membrane destabilization. Over its hundreds of years of use, iodine hasn't elicited bacterial resistance, a trait that's due perhaps to its broad mechanism of action: It may have too many mechanisms for bacteria to adapt to.2 In addition to the oxidation of reactive moieties on cell surfaces, iodine serves to poison the electron transport chain that all living cells use to produce energy. Although there may be some way for a cell to develop resistance by preventing contact with extracellular iodine, it is difficult to imagine what form such a mutation could take. To develop resistance to electron transport, the cell would need to evolve properties that would no longer be consistent with the definition of living organisms. The evolution of bacterial resistance towards electron-electrophile reactions seems exceedingly unlikely.
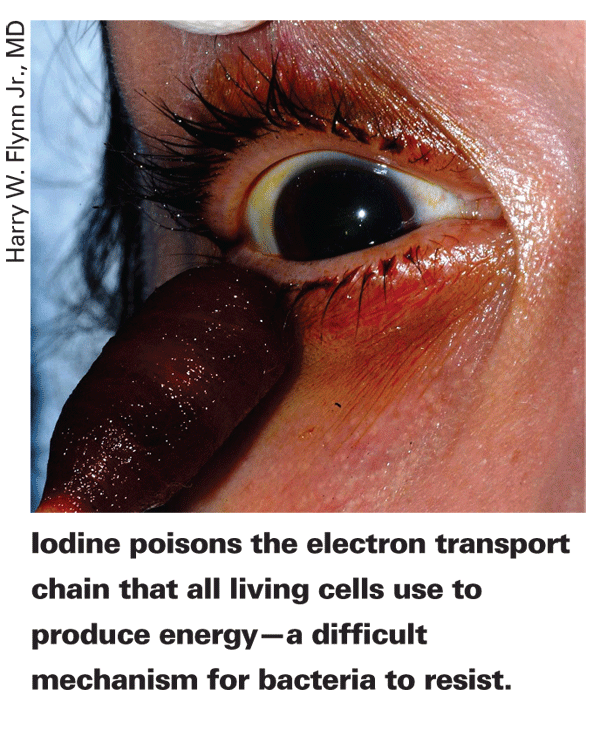
Lugol's 5% iodine/10% potassium iodide solution contains a high level of free molecular iodine and is therefore highly potent and must be used with caution for fear of staining and toxicity. Also, it shouldn't be applied to open wounds, as strong iodine solutions can cause unpleasant stinging and allergic skin reactions. In 2007, the usefulness of iodine crystals in methamphetamine production became apparent. The sale of iodine crystals and strong iodine solutions (greater than 2.2%) is now regulated by the Drug
Enforcement Administration, and records must be kept detailing the amount sold and the address of the purchaser.4 Iodophor solutions such as PVP-I, whose complexed iodine prevents its extraction by clandestine drug operations, are exempt from the regulation.5
The toxicity of Lugol's solution necessitated the development of alternative, less-irritating solutions that maintained a high degree of antimicrobial activity. In addition to problems associated with local toxicity, the application of iodine as a useful antiseptic has always been complicated by chemical instability and poor water solubility. Today, the pure aqueous and alcohol solutions of iodine have largely been replaced by iodophor solutions, which employ organic complexing agents to achieve certain effects.
In the case of PVP-I, it contains the neutral polymer polyvinylpyrrolidone in order to improve its stability, toxicity and solubility. The concentration of iodine in a solution, and, particularly, the amount of free iodine, is directly related to its antimicrobial capacity.
It's this free molecular iodine that correlates to bactericidal activity. Solutions with the same total concentration of iodine but different amounts of free iodine vary greatly in their antiseptic abilities.6
Maximizing Efficacy
Iodine is the only agent that is consistently active against gram positive and gram negative bacteria, spores, amoebic cysts, fungi, protozoa, yeasts, drug-resistant bacteria such as MRSA3 and viruses.7-9 Interest is keen, therefore, to minimize its side effects and maximize its killing efficacy. Ocular irritation caused by PVP-I occurs when solutions with high levels of free molecular iodine and moderate to high levels of total iodine are used. High concentrations of free molecular iodine are tolerated in the eye if the total iodine content is very low, as the amount of active agent is generated at a controlled and continuous rate over its life.10 In-vitro analysis of PVP-I reveals that dilution of PVP-I solutions produce surprising results, with dilutions from 1:2 to 1:100 producing a more rapid kill of S. aureus and M. chelonae than stock 10% solution.11 The chemistry reveals that the amount of free iodine increases until an approximate 0.1% dilution is reached; thereafter, the solution behaves as a non-iodophoric aqueous solution (e.g., iodine-water or iodine-alcohol solutions) with a much slower killing rate.3 This trend has not been consistently replicated in vivo, and seems to be related to a variety of factors, including the initial bacterial load, any binding to organic substances such as blood, pus and fat,13 and the method and duration of application. While in-vitro tests with PVP-I rely on its culture activity, in-vivo analyses require the iodine to interact with all the components of the skin and ocular surface, when only the living microorganisms are of concern. These unwanted interactions consume the available free iodine.12
A few clinical studies have attempted to demystify the in-vivo activity of various dilutions of PVP-I. One study randomized pre-cataract surgery patients to receive preoperative irrigation with either 1% or 5% PVP-I, and the researchers then took cultures both before and a minute after irrigation. The study doctors instilled 2 mL of solution into the eye over a minute. They ultimately obtained a significantly larger decrease in isolates with a 5% PVP-I solution than with a 1% solution.14 Another study subjected healthy canine eyes to a two-minute scrub and two-minute soaking procedure using 1:2, 1:10, or 1:50 dilutions of a 1% PVP-I solution. It found that the bacterial growth that was initially detected in 32 of the 46 eyes was not detectable after the scrub-soaking procedure, regardless of the dilution used.15 Using a one-drop technique that instilled a drop of 5% PVP-I in one preoperative eye and a drop of 0.02% PVP-I in the contralateral eye, researchers found that viridans streptococci and micrococci cultures were similar.16
The administration guidelines for Alcon's Betadine (povidone-iodine) 5% Sterile Ophthalmic Prep Solution describe saturation of the lids, brow and cheek in ever-widening circles until the entire surgical field is covered. The cornea, conjunctiva and palpebral fornices are then to be irrigated and left in contact with the solution for two minutes prior to flushing with a sterile saline solution. As none of the studies discussed above precisely replicated this application technique, conclusions about the in-vivo efficacy of higher dilutions of PVP-I remain uncertain.
Ocular Use
In the
Unfortunately, the low incidence of endophthalmitis infections makes research into effective preventive measures difficult. It appears that a flush with povidone iodine (e.g., 5%) is more effective at reducing conjunctival colonization than several drops of the same strength solution. Irrigation dislodges the bacteria lying in wait in the fornix, eyelid margin and eyelashes. These results are only significant, however, when culture organisms are isolated in a liquid media, since plated cultures don't show any significant difference.18 These colonies, however, are not necessarily indicative of a clinical infection. The amount or presence of bacteria that causes an infection in one person may be entirely different for someone else. The development of a postoperative infection may be a more clinically relevant endpoint, but the numerous factors involved make causation difficult to demonstrate.
Despite the vagaries, the efficacy of PVP-I at reducing the risk of infection extends beyond endophthalmitis. In general hospital admissions, PVP-I is part of a highly effective regimen that also consists of various antibiotics (depending on the region affected), chlorhexidine and octenidine for decolonizing patients afflicted with methicillin-resistant Staphylococcus aureus (MRSA).19 A controlled trial also revealed that q.i.d. povidone-iodine 1.25% was as effective as neomycin-polymyxin-B-gramicidin for treating bacterial conjunctivitis. It was also as effective at treating viral conjunctivitis, though neither the antibiotic nor the povidone solution reduced the clinical course of the disease.20 While antibiotics are the most effective treatment for corneal ulcers, PVP-I solutions have been suggested as a useful adjunctive therapy for more stubborn cases.21 For cultured human corneal epithelial cells, PVP-I demonstrates higher antimicrobial activity and less cytotoxicity than hydrogen peroxide, polyhexamethylene biguanide and benzalkonium chloride, suggesting a potential use as a contact lens disinfectant.22 It also can be used as a prophylactic agent against ophthalmia neonatorum,23 and may even facilitate wound healing in chronic, non-healing wounds through a reduction in metalloprotease activity.24
The issue of PVP-I's effect on wound healing is an intriguing one. Persistent reports in the literature tell of delayed wound healing potentially stemming from PVP-I application. Cell proliferation assays with cultured fibroblasts suggested that PVP-I reduces the migration and proliferation of fibroblasts in a dose-dependent fashion.25 But again, we encounter the in-vitro versus in-vivo issue: Human studies sometimes implicate iodine as an accelerant in the wound healing process.24
If there's one thing the literature agrees on with regard to PVP-I, it's that it's vital in reducing the risk of intraoperative infections, including endophthalmitis. PVP-I's ability to indiscriminately reduce bacterial and microbial flora certainly offers an invaluable and impressive line of defense, despite the quarrels over the most effective solution and dilution for it. Currently, the safest and most effective protocol for the use of PVP-I includes the instructions dictated by the package insert. Soon, we may see PVP-I's antimicrobial activity and low cytotoxicity result in its use in contact lens solutions, preservatives and novel anti-infectives for the skin, ear and eye.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Marquis RE, Clock SA, Mota-Meira M. Fluroide and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev 2003;26:5:493-510.
2. Selvaggi G, Monstrey S, Van Landuyt K, et al. The role of iodine in antisepsis and wound management: A Reappraisal. Acta Chir Belg 2003;103:2 1-247.
3. Gottardi W. Iodine and Iodine Compounds. In: Block S, ed. Disinfection, Sterilization, and Preservation.
4.
5. Drug Enforcement Administration. 21 CFR Parts 1309 and 1310. Changes in the regulation of iodine crystals and chemical mixtures containing over 2.2 percent iodine. Available at http://www.deadiversion.usdoj.gov/fed_regs/rules/2007/fr0702.htm. Accessed 27 February 2009.
6. Hickey J, Panicucci R, Duan Y, et al. Control of the amount of free molecular iodine in iodine germicides. J Pharm Pharmacol 1997;49:12:1195-9.
7. Isenberg SJ, Apt L. The ocular application of povidone-iodine. Community Eye Health 2003;16:46:30-1.
8.
9. Solka D, Hermonat PL. Inactivation of papillomavirus by low concentrations of Povidone-iodine. Sex Transm Dis 1995;22:22–4.
10. Duan Y, Dinehart K, Hickey J, et al. Properties of an enzyme-based low level iodine disinfectant. J Hosp Infect 1999;43:219-29.
11. Berkelman RL,
12. Gottardi W. The influence of the chemical behaviour of iodine on the germicidal action of disinfectant solutions containing iodine. J Hosp Infect 1985;6(Suppl):1-11.
13.
14. Ferguson AW, Scott JA, McGavigan J, et al. Comparison of 5% povidone-iodine solution against 1% povidone-iodine solution in preoperative cataract surgery antisepsis: A prospective randomized double blind study. Br J Ophthalmol;2003;87:163-7.
15. Roberts SM, Severin GA, Lavach JD. Antibacterial activity of dilute povidone-iodine solutions used for ocular surface disinfection in dogs. Am J Vet Res 1986;47:6:1207-10.
16. Grimes SR, Hollsten D, Nauschuetz WF, et al. Effect of povidone-iodine irrigation on the preoperative chemical preparation of the eye. Mil Med 1992;157:3:111-3.
17. Thoms SS,
18. Safar A, Dellimore MC. The effect of povidone-iodine flush versus drops on conjunctival colonization before intravitreal injections. Int Ophthalmol 2007;27:307-12.
19. Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol 2008;29:510-6.
20. Isenberg SJ, Apt L, Valenton M, et al. A controlled trial of povidone-iodine to treat infectious conjunctivitis in children. Am J Ophthalmol. 2002;134:681-8.
21. Hale LM. The treatment of corneal ulcer with povidone-iodine (Betadine). NC J Med 1969;30:2:54-6.
22. Yanaia R, Yamadaa N, Uedaa K, et al. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: Antimicrobial activity and cytotoxicity for corneal epithelial cells. Cont Lens Ant Eye 2006;29:2:85-91
23. Najafi RB, Samani SM, Pishva N, Moheimani F. Formulation and clinical evaluation of povidone-iodine ophthalmic drop. Iranian J Pharm Res 2003;2:157-60.
24. Eming SA, Smola-Hess S, Kurschat P, et al. A novel property of povidone-iodine: Inhibition of excessive protease levels in chronic non-healing wounds. J Invest Derm 2006;126:2731-3.
25. Gregory T, Rael LT, Bar-Or R, et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 2009;66:1:82-91.



