Two New Tests
Researchers at the Ocular Genomics Institute (associated with the Massachusetts Eye and Ear Infirmary and Harvard Medical School in Boston) have developed a CLIA-certified, next-generation, gene-sequencing protocol designed for patients with inherited eye diseases, including glaucoma, retinal degenerations and optic atrophy. The tests are referred to as Genetic Eye Disease panels, or GEDi. The GEDi-R test looks for mutations relating to retinal disorders; the GEDi-O test checks for mutations relating to optic atrophy and early onset glaucoma.
Clinical testing results, reported in a recent publication,1 have demonstrated that the GEDi tests’ ability to detect a single nucleotide variant has a sensitivity and specificity of 97.9 percent and 100 percent, respectively. (The study authors note that this compares favorably with the 88.3-percent sensitivity achieved by whole-exome sequencing using a commercially available exome capture set; they attribute this to better coverage of targeted genes in the GEDi tests.) Prospective testing of 192 patients with inherited retinal degenerations found that the retinal GEDi test had a diagnostic rate of 51 percent.
These tests can be ordered by a medical professional; turnaround is 90 days. The retinal test costs $2,500; the atrophy/glaucoma test costs $1,250. (Health insurance may cover part or all of the cost.)
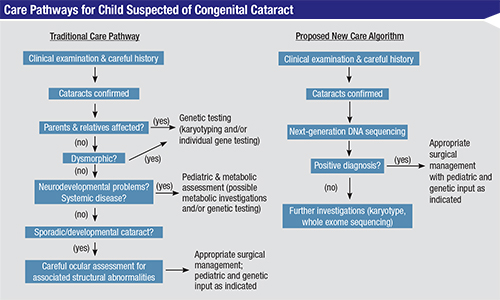
Another recent development is an advanced DNA test relating to congenital cataracts, a condition that can be a symptom of more than 100 different diseases. Uncovering the mutations linking the congenital cataracts to those diseases used to be a long, costly and not totally reliable process involving multiple genetic tests and numerous non-genetic tests, guided by a detailed family history. Now, researchers at the University of Manchester in England have developed a test using a new DNA-sequencing technology called next-generation sequencing, or NGS. The new test looks at 115 genes known to be associated with congenital cataracts and can find mutations connected to one of those diseases within a few weeks. (The new test has also uncovered mutations related to the condition that were not previously known.)
“Congenital cataract is a difficult condition to diagnose genetically; more than 100 genes have been associated with it,” notes Rachel Gillespie, who designed the new test and is lead author of the study. “Importantly, cataracts in children and babies can present as an isolated problem or as an early indication of an underlying multi-system condition. However, clinical presentations in infants and young children can be very mild and ambiguous, so delineation of the precise cause is almost impossible. [At the same time,] prompt diagnosis of these conditions is imperative so that early preventative treatment and/or disease monitoring can commence as soon as possible.
“Traditional genetic testing methods would require screening of cataract-causing genes individually and consecutively to find the cause—a process that can take a very long time and is often unsuccessful,” she continues. “Our test screens 115 cataract genes simultaneously from a single small blood or saliva sample, making diagnosis much easier and more efficient. We have seen a very high diagnosis rate: Our test is able to find the likely cause in about 75 percent of all patients tested. Interestingly, for a number of children, our genetic findings have enabled a diagnosis of specific conditions, altering their clinical management and treatment. Furthermore, identification of the genetic cause of congenital cataract within families enables counseling for prognosis, the risk to other family members and advice on prenatal testing in future pregnancies.
“For example, one family we worked with had three members—two brothers and their cousin—who presented with childhood-onset cataracts, seizures and challenging behavior with autistic features that seemed to be worsening with age,” she says. “They had each undergone numerous tests to try and determine the cause, and despite additional findings of delayed myelination from an MRI scan, a precise diagnosis was not made. NGS genetic screening identified a mutation in the gene CYP27A1 that is known to cause cerebrotendinous xanthomatosis, a lipid-storage disorder that can be fatal; we confirmed this mutation as pathogenic by lipid profiling. CTX is very mild in infancy (initial presentations are cataract and diarrhea), but becomes much more serious with age. Early diagnosis is crucial because preventative treatment is available in the form of chenodeoxycholic acid and statins, which may prevent disease progression but cannot reverse it later on. Luckily, we were able to diagnose this condition relatively early in this family and they are all doing well on treatment.”
Ms. Gillespie says they’ve been working hard on this new technology. “We’re currently researching the impact this test is having on the care of congenital cataract patients,” she says. “The test has been available in the U.K. since December 2013, and it can be requested by registered medical facilities via international referral on a diagnostic (rather than research) basis. Referral information can be found at mangen.co.uk, along with sample criteria. To conduct the test we ask for either a minimum of 1 ml of blood in EDTA (Ethylenediaminetetraacetic acid), or 10 µg of high-quality DNA.”
Making Testing Accessible
Another laboratory doing notable work is The John and Marcia Carver Nonprofit Genetic Testing Laboratory, affiliated with the University of Iowa. The lab, headed by Edwin M. Stone, MD, PhD, and Val C. Sheffield, MD, PhD, is dedicated to providing non-profit genetic testing for rare eye diseases. The tests they offer incorporate the research done by Drs. Stone and Sheffield, so the tests provide the most clinically relevant information while remaining affordable.
“When I started working for the Carver Lab there were probably 20 inherited eye disease genes known,” says the lab’s Jean Andorf. “Now there are more than 250. So the field has grown fast, and our testing has grown with that gene discovery rate. We were motivated to offer these services because once a gene has been found and studied for a long time you can’t take grant money for the purpose of genotyping more families. So, all these research dollars would go to discover a gene, and then the genetic testing wouldn’t be available for the patients. Our goal was—and still is—to offer affordable genetic testing to anyone who wants it. What we charge for a test is truly just the cost of the lab technicians and the reagents.
“We are also a research lab,” she continues. “About a third of our effort focuses on nonprofit genetic testing; and about two-thirds is on research. We’re constantly looking for new genes, as well as ways to better understand the genes that are known. For example, one of our biologists, Budd Tucker, PhD, is making huge strides in using pluripotent stem cells to treat people with inherited eye diseases.”
One of the projects under way at the Carver Lab is referred to as “Project 3,000,” an effort to identify every person in the United States suffering from Leber’s congenital amaurosis—estimated to be about 3,000 in number. “This program has allowed us to offer genetic testing to these individuals and populated a number of the RPE65 treatment trials, while providing a significant population for future clinical research into long-term prognosis,” says Ms. Andorf. “In addition to finding most of the LCA patients under the age of 20, we’ve also identified a handful of older people with the disease. Some didn’t realize they had this disease; many were simply born blind during an era when little was known about inherited eye diseases. Many of these adults are cognitively normal with high intelligence and functioning very well. That enables us to give hope to families with a child sharing that particular genetic cause. You can say, ‘I have a 70-year-old patient who did just fine in her life who has the same type of genetic mutation as your child.’ That kind of information is hugely beneficial for a family, even if it doesn’t bring them treatment today.”
Making the Most of Testing
Ms. Andorf offers several suggestions regarding genetic testing:
• Your patient may not need complete exome testing. “Thanks to the existence of exome sequencing, many laboratories will simply give you all of the data they find,” she notes. “However, there are several diseases that are caused by one small gene or just a few genes. A whole exome costs several thousand dollars, whereas a test for that one mutation may cost a couple hundred dollars. While exome sequencing certainly has a place in genetic testing for inherited eye diseases, it’s not a good use of anyone’s resources to do a complete exome sequencing for a person with a monogenic disease.”
Ms. Andorf says a lot of unnecessary testing results from physicians not trusting their own diagnosis. “We really want doctors to order the right test,” she says. “It’s not good for us if a doctor orders multiple tests on our website for a patient because he’s trying to find a diagnosis. Doctors have been seeing most of these patients for a long time; they need to trust their clinical expertise to try to match the patient’s clinical findings with the appropriate test. Our goal is not to diagnose the patient through testing; our goal is to confirm the diagnosis. In fact, we’re working on sharing clinical information with physicians that will help them narrow down the testing for their patients.”
Ms. Andorf says that for this reason, they often screen patients who have heterogeneous diseases in tiers. “We start by testing for the most likely genetic mutation,” she explains. “In a significant percentage of patients we identify the mutation with a single, inexpensive test using this protocol. The other advantage of this approach—which some people criticize us for—is that a complete exome reveals a lot of irrelevant information that can cloud a diagnosis. We screen the gene most consistent with your clinical features.
“It’s even more important to have a diagnosis that’s as accurate as possible when pursuing exome sequencing,” she adds. “On average, exome sequencing will reveal very plausible disease-causing mutations in eight known inherited eye disease genes. Thus, patients with inaccurate diagnoses will often have misleading findings in genes consistent with the inaccurate diagnosis. We’ve seen cases in which families and physicians have been misled because of this type of situation. Too much genetic information can make the results harder to interpret.”
• Make sure the patient is consulting with a genetic counselor. “Lots of patients e-mail us reports that they got from another lab—a list of mutations with no interpretation of the findings,” says Ms. Andorf. “Here, we work with an inherited eye disease specialist who has dealt with inherited eye disease for more than 25 years. Whatever disease you’re dealing with, we’ve probably screened thousands of others with the same disease. Having the ability to see clinical correlations to genetic test results gives you a higher level of confidence in the interpretation and minimizes information that may be confusing.
“For that reason, the physician requesting a test from us has to write down who is providing genetic counseling to the patient,” she says. “Patients often want us to send the report directly to them. We say, ‘If your doctor ordered a kidney function test for you, the lab wouldn’t send the results directly to you. You really need to have a physician and a genetic counselor to be able to understand these data.’ We try to get the physician and family to think about that in advance of the test.”
• Turnaround time should not be your only consideration. “We are sometimes criticized for our long turnaround times,” notes Ms. Andorf. “That’s true in some cases, but we group patients together in order to keep the costs as low as possible. And we’re not just a clinical laboratory. Some services will offer a complete exome sequencing within four weeks, but we believe it makes more sense to order a specific test that’s in line with your own diagnosis, even if it takes longer to receive the results.”
Locating Resources
As the number of tests available increases, along with the number of laboratories offering the tests, the need for a central clearinghouse has become evident. One company attempting to meet that need is GeneTests,
based in Elmwood Park, N.J. Its mission is to promote the appropriate use of genetic testing by providing current, easy-to-access, free information about test availability.
According to Deborah L. Eunpu, manager at the company, the most commonly requested genetic tests relating to eye disease include tests for Leber’s congenital amaurosis; optic atrophy; retinitis pigmentosa; retinoblastoma; age-related macular degeneration; oculocutaneous albinism; congenital cataracts; congenital glaucoma; malformations of the eye (e.g., aniridia, microphthalmia, anophthalmia); and dislocated lens. Ms. Eunpu says that once a surgeon has found a laboratory that offers the services in which the surgeon is interested, he can contact the lab directly.
|
“A lot of unnecessary testing results from physicians not trusting their own diagnosis. Our goal is not to diagnose the patient ... [but] to confirm the diagnosis.”
— Jean Andorf |
Ms. Eunpu notes that test usage is increasing, and options for testing continue to expand. “In the past year we’ve added nearly 10,000 new tests, many due to new technologies,” she says. “For example, the availability of next-generation sequencing has opened the door to testing multiple genes at a time. These tests can be most helpful if one is not sure which of several related conditions to test for. However, when a specific diagnosis is suspected, a single gene can be interrogated.”
Ms. Eunpu expects to see even more new tests and increased usage. “Genetics continues to be an exciting, evolving field in which advances in technology and knowledge can lead to rapid changes,” she says. “Will everyone have full sequencing? Not likely. But as more treatments are based on knowing the specific genetic mutation, the reasons to do many tests will be compelling. With clinical trials and opportunities for improved vision arising through emerging treatments, testing is being looked at much differently. Now testing may lead to specific treatments.” REVIEW
1. Consugar MB, Navarro-Gomez D, Place EM, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med. 2014;Nov 20. [Epub ahead of print.]
2. Gillespie RL, O’Sullivan J, Ashworth J, et al. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology 2014;121:2124-2137.