IOP is a dynamic physiological parameter that doesn't remain constant over the course of 24 hours. Trough IOP levels tend to occur at the end of the waking period; peak IOP is usually recorded at the end of the nocturnal sleep period.1,2 This is true for both normal subjects and untreated glaucoma patients. (See chart, below.) In addition, another important event occurs simultaneously when we are in the supine position during sleep: a drop in systemic blood pressure. 
John H. K. Liu, PhD
The combination of high IOP and low systemic blood pressure can cause a critical reduction in ocular perfusion pressure. In susceptible eyes, this can lead to the progression of glaucomatous damage. Additionally, studies suggest that large fluctuations in 24-hour IOP may be a significant risk factor for glaucoma progression.3 Therefore, it makes sense to do as much as possible to control both diurnal and nocturnal IOP.
Typically, we monitor a glaucoma patient's IOP during daytime hours, usually with the patient in an upright, sitting position. Most of us have little or no information about our patient's IOP during the nocturnal period, which accounts for roughly one-third of the 24-hour cycle. Given the danger posed by IOP fluctuations at night, it makes sense to learn more about the way IOP fluctuates over time in our patients and evaluate how well our treatment protocol is controlling IOP when the patient is not in the office.
Clinically speaking, how much difference would it make to know what's happening with a given patient's IOP during the night? One study showed that 24-hour IOP monitoring led to changes in clinical management in almost 80 percent of the subjects.4 Clearly, knowing how our treatment is affecting nocturnal IOP could be an issue in the management of many of our glaucoma patients.
Controlling IOP at Night
What causes the dramatic increase in pressure seen in some patients during the nocturnal hours? The main cause is the change in episcleral venous pressure that occurs when the patient is in a supine position. However, this factor alone doesn't account for all of the rise in nocturnal IOP.
We're not yet certain what all the contributing factors are, but studies of aqueous flow rates have shown that aqueous production during sleep decreases to about half of daytime levels.5,6,7 Thus, aqueous production is not likely to be contributing to the rise in IOP. This suggests that an increase in outflow resistance may be involved.
Most of our IOP-lowering meds function either by reducing aqueous production or increasing aqueous outflow. The beta blockers, carbonic anhydrase inhibitors and alpha agonists decrease aqueous production; the prostaglandin analogues increase uveoscleral outflow. How well do the different drug classes control IOP during the nocturnal period when pressure tends to rise? Fortunately, well-conducted studies have looked at this issue with several medications:
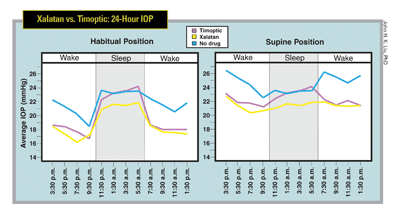
• Beta blockers. As a recent study confirmed, beta blockers are known to have decreased efficacy during the nocturnal sleep period.8 The explanation for this may have something to do with the possibility that beta blockers block the effects of endogenous catecholamines, which drive aqueous humor formation in the ciliary epithelia. As the endogenous levels of epinephrine and other catecholamines decrease during sleep, there may be less of an opportunity for beta blockers to exert their effects.9 This may affect aqueous flow rates.
• Prostaglandin analogs. These have been shown to decrease IOP throughout the 24-hour cycle. This treatment effect is similar for both latanoprost and bimatoprost.
• Carbonic anhydrase inhibitors. CAIs have been found to decrease IOP throughout the 24-hour cycle as well, although the effect is much less pronounced than that seen with the prostaglandins and prostamides.
• Alpha-2 agonists. There is a paucity of data looking at brimonidine during the nocturnal sleep period, although many studies have confirmed its efficacy during the day. However, a recent study looked at the 24-hour IOP profiles of patients on brimonidine Purite vs. dorzolamide added to latanoprost, and found similar efficacy in both groups.10
John H. K. Liu, PhD
Given the superior efficacy and low side-effect profile, I usually start with a prostaglandin as a first-line treatment. If this is not an option for a given patient, the combination of timolol and dorzolamide usually produces a considerable IOP reduction, and has been found to have a sustained IOP reduction throughout the 24-hour period.
If medical treatment doesn't produce a satisfactory result, argon laser trabeculoplasty has also been shown to decrease IOP throughout the 24-hour cycle (article in press), and is another treatment option to consider.
Tracking IOP Around the Clock
Ideally, to know which patients are undergoing the most dramatic IOP increases at night, and to monitor the effectiveness of our treatment, we'd need to check the patient's IOP periodically throughout the night. Unfortunately, 24-hour IOP testing is impractical for the majority of our patients, so it's very difficult to obtain this sort of information.
In particular, determining whether a specific treatment not only controlled IOP during sleep but also caused less glaucoma progression in a population of subjects would require repeated 24-hour testing over a period of months or years. Furthermore, many patients are on multiple systemic medications; these may affect an individual's response to various treatments. Until we develop reliable, user-friendly home tonometry or (better yet) continuous IOP monitoring devices, this information will remain largely unavailable to us.
However, the availability of such devices may be just around the corner. Multiple investigational devices— implantable continuous IOP monitors and external tonometers—are already being developed. These will not only give us more information regarding our patients' 24-hour IOP profiles, but more importantly, may allow us to individualize treatments based on patient responses to different treatment modalities, as opposed to applying generalized knowledge to individual patients.
Estimating Nocturnal IOP
Although it's not convenient to monitor a patient's nocturnal IOP in most cases, a study I conducted with John H.K. Liu, PhD, and Robert N. Weinreb, MD, demonstrated that it's possible to estimate the peak nocturnal IOP of some patients based on readings taken in the office.11
We reviewed 24-hour IOP data collected in a sleep laboratory from three groups of patients of different ages: 33 healthy subjects aged 18 to 25 years; 35 healthy subjects aged 40 to 74 years; and 35 untreated glaucoma patients aged 40 to 79 years. During the waking period, IOP was measured every two hours using a pneumotonometer in the sitting and supine positions; measurements were also taken in the supine position during the nocturnal/sleep period. We looked for correlations between measurements taken in either position during office hours (9:30 a.m. to 3:30 p.m.) and peak IOP during the nocturnal period.
Although we found no correlation in the younger, healthy subjects, and only a small correlation in older, healthy subjects, we found a significant correlation between peak nocturnal IOP and average supine IOP measurements taken during office hours in the older glaucoma subjects (r = .713, p<.001). This suggests that the magnitude of peak nocturnal IOP in untreated glaucoma patients can be estimated based on supine IOP measurements taken during routine office visits.
Having an estimate of a new glaucoma patient's peak nocturnal IOP may allow us to better choose treatment and prevent the deleterious effects associated with IOP spikes occurring outside of office hours.
Dr. Mosaed is assistant professor of ophthalmology at the University of California, Irvine.
1. Liu JH, Kripke DF, Twa MD, et al. Twenty-four hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci 1999;40:2912-2917.
2. Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci 2003;44:1586-1590.
3. Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000 9:2:134-42.
4. Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12:3:232-6.
5. McLaren JW, Trocme SD, Relf S, Brubaker RF. Rate of flow of aqueous humor determined from measurements of aqueous flare. Invest Ophthalmol Vis Sci 1990;31:2:339-46.
6. Brubaker RF. Flow of aqueous humor in humans [The Friedenwald Lecture] Invest Ophthalmol Vis Sci 1991;32:13:3145-66. Review.
7. Maus TL, McLaren JW, Shepart JW Jr, Brubaker RF. The effects of circulating catecholamines and aqueous flow in human subjects. Exp Eye Res 1996;62:351-358.
8. Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol 2004;138:3:389-95.
9. Rettig ES, Larsson LI, Brubaker RF. The effect of topical timolol on epinephrine-stimulated aqueous humor flow in sleeping humans. Invest Ophthalmol Vis Sci 1994;35:2:554-9.
10. Konstas AG, Karabatsas CH, Lallos N, Georgiadis N, Kotsimpou A, Stewart JA, Stewart WC. 24-hour intraocular pressures with brimonidine purite versus dorzolamide added to latanoprost in primary open-angle glaucoma subjects. Ophthalmology 2005;112:4:603-8.
11. Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol 2005;139:2:320-4.




