In Chinese philosophy, it's believed that most things in the world, including humans, possess complementary dark and light aspects, a negative and a positive, known as the yin and yang. In one organism at least, the adenovirus, the concept of yin and yang is especially relevant. While adenoviruses are the most common cause of viral conjunctivitis and viral corneal infections in the world,1 they may also hold the key to treating such conditions as cystic fibrosis, muscular dystrophy, cancer and retinal dystrophies. In this article, we'll explore the dark and light sides of this complex organism.
The Adenovirus
Adenoviruses comprise a group of about 50 DNA viruses that may account for up to 66 percent of viral cases of conjunctivitis.2 Respiratory tract infections, atypical pneumonia, gastroenteritis and urethritis can also result from adenoviral infection and have been associated with adenoviral conjunctivitis.3,4 During an adenoviral infection, the virus enters human cells and alters their gene expression to bring on the disease state. In the early mode of viral transcription, the adenovirus targets the transcriptional factors of E1A proteins, such as p300/CBP, which activate cell proliferation, and of E3 proteins, which interfere with the inflammatory response. Within 36 hours of infection, adenovirus DNA is replicated to instigate viral gene expression and hinder cellular gene expression. By the end of the second day of infection, cellular genes are increasingly down-regulated, resulting in organelle dysfunction.5 Symptoms of adenoviral infection—which include irritation, lacrimation, discomfort, and sometimes fever, photophobia, punctate keratitis, inflammation of the pretragal nodes, and follicular conjunctivitis—will begin to show about a week after infection. Infiltrates appear after 11 days of infection, and sizes and numbers will vary with the adenovirus type. Even after symptoms have cleared, adenoviruses can remain in the body for years.1
As malevolent as the adenovirus seems, the agent has the capacity for benevolence, particularly as a therapy for ocular diseases and other illnesses unrelated to the eye.6-9 The mechanism through which adenoviruses transfect cells can be manipulated to facilitate the delivery of useful genes into cells, thereby halting or controlling a disease state. Ironically, the development of vector therapy has given these agents a new, better reputation—a yang to balance the yin of the adenoviral disease-causing vector.
Ocular Adenoviral Infections
Ocular adenoviral infections primarily present as pharyngoconjunctival fever, epidemic keratoconjunctivitis, acute nonspecific follicular conjunctivitis or chronic adenoviral keratoconjunctivitis. Adenoviral keratitis has been known to occur in post-LASIK patients.10, 11 PCF, most common in children, is usually caused by adenoviruses serotype 3, but it has also been associated with adenoviruses serotypes 1, 4, 5, 6, 7 and 14. After an incubation period of five to 12 days, the patient will experience a fever for approximately 10 days. This infection is bilateral and is also associated with a sore throat; additional symptoms consist of slight itching and burning, definite irritation and tearing and some photophobia. The patient may also suffer from swollen eyelids during the first two days. Other symptoms may include serous discharge and slight crusting on the lids, which is probably bacterial. About a week after the onset of symptoms, punctate keratitis may develop, which can lead to subepithelial infiltrates in the cornea. PCF typically resolves itself in a few days to three weeks.1
EKC was originally known as shipyard eye, a testament to the probable reuse and poor sterilization procedures of instruments used by surgeons to remove foreign bodies from the eyes of workers in shipyards. This name became widely known in the summer of 1941, when an outbreak occurred in Pearl Harbor, and more than 10,000 people became infected with the malady.12 Eventually less sensationally renamed as epidemic keratoconjunctivitis, it's a more serious ocular manifestation that is usually associated with adenovirus serotype 8.
EKC can be transmitted by contaminated ophthalmic instruments during non-sterile eye examinations,13 and serotype 19 can infect others via close personal contact, including sexual activity. Following an eight-day incubation period,1 tears and saliva are contagious for about two weeks.14 In cases of severe conjunctival involvement, subconjunctival hemorrhaging occurs and pseudomembranes form, inducing moderate discomfort. Keratitis occurs in 80 percent of patients, who will also be afflicted with discomfort, photophobia, tearing and mild blepharospasm. A live adenovirus causes superficial epithelial punctate keratitis, which by day 11 develops into subepithelial white corneal lesions and a significantly red eye. In three to four weeks, the conjunctivitis and keratitis will gradually resolve. Nonetheless, the residual corneal subepithelial opacities and photophobia can last up to a year.
Environments in which the adenovirus can easily spread include industrial areas, swimming pools and hospitals; the last contains the risk factors of ophthalmic instruments—especially tonometers—and ophthalmic solutions as fomites for the virus. EKC usually occurs in young adults in the fall and winter season, and it is unilateral in two-thirds of cases.1
Acute nonspecific follicular conjunctivitis doesn't involve the cornea, but presents with red eyes and pretragal node inflammation, and is typically only seen by pediatricians and family physicians. This form of adenoviral infection usually resolves itself in seven to 10 days, but it can lead to an epidemic. Chronic adenoviral keratoconjunctivitis is a much rarer form of adenoviral disease that involves intermittent exacerbation of tearing, redness and photophobia, as well as the presence of acute conjunctivitis several months preceding onset.1
For the most part, adenoviral infections are self-limiting, but treatment is still beneficial for enhanced recovery time and for the reduction of discomfort. Untreated, these conditions can last for up to several years.1 Diagnostic challenges hinder clinicians from providing the speediest diagnosis and earliest appropriate treatment possible. The clinical characteristics of adenoviral conjunctivitis may have some similarities to those of herpes simplex virus conjunctivitis; consequently, misdiagnosis occasionally occurs. One study demonstrated that 13 percent of clinically diagnosed herpes-related conjunctivitis cases, when laboratory tested, were actually caused by adenovirus. Likewise, 4.8 percent of diagnosed adenoviral conjunctivitis infections were, in fact, herpes conjunctivitis infections.2 Another study similarly indicated that 4.3 percent of cases of clinically diagnosed EKC were actually caused by herpes.15 Cases such as these point to the importance of testing conjunctival secretions or blood samples in order to confirm diagnoses.
Conjunctival scrapings that exhibit lymphocytes, PMN infiltrates and degenerated epithelial cells without inclusion bodies would indicate an adenoviral infection. Proof of diagnosis requires a paired blood specimen, with one taken a week after symptom onset and another taken two to three weeks later. A fourfold or greater increase in humoral antibody to adenovirus confirms the diagnosis. Other diagnostic tests include immunofluorescence and an enzyme-linked immunosorbent assay. Even with accurate diagnosis, treatment options are controversial and remain limited.1
Treatment
Though ocular adenoviral infections usually resolve themselves, some cases can take several years for all the symptoms to fully disappear, and no FDA-approved drug is available for the treatment of adenoviral infections. Of the researched treatment options for adenoviral disease, antiviral drugs appear to have the most promise, yet they are still far from optimal. Antiviral treatment is most effective when it's used during the replicative phase of the adenovirus. Unfortunately, since this occurs before the clinical appearance of keratoconjunctivitis, the small window of opportunity for effective antiviral therapy is often missed. Still, in cases of early diagnosis, antiviral prophylaxis in the forms of the agents ribavirin and cidofovir show promise in preventing the spread of the adenovirus.16, 17
A preclinical study on ribavirin, a nucleoside antimetabolite that hampers the duplication of viral genetic material, revealed the drug's potential as an antiviral medication.17
A clinical study demonstrated that cidofovir—an antiviral drug that selectively inhibits viral DNA synthesis—is effective in decreasing the number of severe corneal opacities. Nevertheless, the high dosage of cidofovir required for efficacy also produces local toxicity,16 and the drug is currently only indicated for the treatment of cytomegalovirus retinitis in AIDS patients. Although some studies suggest that steroids or nonsteroidal anti-inflammatory drugs worsen viral infections,18 clinical experience has shown otherwise. Topical steroids temporarily alleviate symptoms of severe conjunctivitis, eliminate the infiltrates, don't prolong the disease and make the condition more tolerable. In cases of pseudomembrane formation, topical antibiotic ointments can protect the cornea.1
Adenoviral Vectors
Although adenoviruses are notorious for being causes of viral conjunctivitis, adenoviral vectors also demonstrate therapeutic potential for ocular inflammatory diseases such as uveoretinitis, corneal allograft rejection and ulcerative keratitis, as well as proliferative vitreoretinopathy, glaucoma, retinitis pigmentosa and macular degeneration. Adenoviral vectors function similarly to lentiviral vectors; both vectors deliver genes to target cells that lack the genetic material dictating the production of a certain protein. As vectors, adenoviruses deliver genes into cells that encode for anti-inflammatory effects or the expression of reporter genes in various ocular tissues such as corneal epithelium, retinal pigment epithelium and the optic nerve.19,20 Vectors can also deliver and disperse genes throughout other tissues, such as within the brains of rats.21
Subsequently, these transduced cells transcribe the recombinant genes to make new proteins, which can ultimately moderate inflammatory processes. Contrary to the mechanism of other viral vectors, such as oncoviral vectors, the recombinant genes introduced by adenoviruses are not incorporated into the DNA of the cell. Therefore, replication continues as normal, and descendant cells do not carry the recombinant genes.21,22 Although this means adenoviral vectors are treatments rather than cures, it also means that cellular reproduction remains normal. Adenoviral vectors are considered safer than other viral vectors because they're less likely to alter normal cellular reproduction.22,23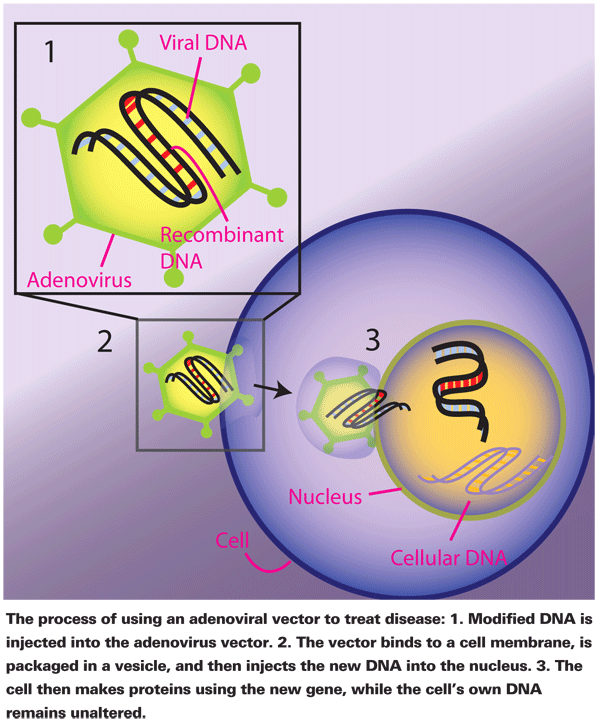
As efficient as adenoviral vectors are, in general it's impossible for them to transfect every single target cell, especially given the constant cycle of cell death and replication that occurs. Repeated treatments are therefore necessary to maintain their effects. Comparatively consistent expression of transfected genes in the retina, however, is feasible since cells in the retina are thought to be non-replicative,24 and the retina's structure and accessibility enable it to be a target organ for genetic therapies.25
Cells transfected with recombinant DNA can continually produce protein.19 Jikui Shen, MD, PhD, of the University of Aberdeen Medical School, and colleagues found adenoviral vectors to be successful in transfecting genes into human conjunctival cells, which were then capable of secreting the anti-inflammatory cytokine vIL-10. This finding implicated adenoviral vectors as a possible treatment for corneal allograft rejection,20 and an ex vivo clinical study has indeed shown adenoviral vectors to be successful in that capacity.26 In an in vitro study of human corneal endothelial cells that were infected with either a mock solution, adenovirus green fluorescent protein (GFP) or adenovirus Simian virus 40 T/t antigen/GFP, the latter recombinant adenoviral vector was the best at enhancing wound healing.27
An adenoviral vector has also demonstrated its ability to inhibit the multiplication of herpes simplex virus in the corneas of mice.28
Notably, a study revealed the exciting finding that an adenoviral vector carrying human retinal pigmented epithelium genes, namely RPE65, restored the vision of dogs with severe retinal dystrophy. Electrophysiological analysis indicated that rod and cone photoreceptor function began as soon as 15 days after the adenoviral vector injection and reached maximal function at three months after the injection. Dogs treated at 8 to 11 months of age retained stable photoreceptor function as a result of adenoviral vector treatment, but the one dog that was treated at age 30 months didn't regain any vision, suggesting that there's a therapeutic window for the delivery of optimal adenoviral vector therapy.29
Another promising therapeutic application of adenoviral vectors is the treatment of proliferative vitreoretinopathy, a disease in which tractional retinal detachment can cause serious vision problems. Gene expression from adenoviral vectors usually lasts less than a month, so early intervention of PVR with soluble type transforming growth factor (TGF-B) inhibition, which diminishes the inflammatory reaction, is more effective in thwarting the disease. In essence, an ounce of early treatment is worth a pound of cure.
A study using rabbits showed that an adenoviral vector transfecting a TGF-B receptor inhibited retinal detachment and the formation of fibrovascular membrane associated with inflammatory cell infiltration. The efficacy and mechanism of these adenoviral vectors are aspects that must be investigated further, as factors other than TGF-B might contribute to PVR. Bear in mind that although adenoviral vectors can bring on moderate inflammatory responses, the magnitude of inflammation can be adjusted by the amount of adenovirus used. Thus, for the best results with these treatments, the researcher has to achieve an optimal dose level to maximize the effectiveness of adenoviral vectors and still minimize inflammation.19
Adenoviruses, a widespread cause of viral conjunctivitis infections, may occasionally plague various populations, but recent research has revealed their benevolent potential to treat syndromes associated with the eye and other diseases. Although adenoviral vectors have been investigated in several in vitro and ex vivo studies on human eyes, the true therapeutic application of adenoviral vectors in humans remains largely a mystery. To our knowledge, no in vivo studies have been performed on human eyes. The yang of the adenovirus, gene transfer using adenoviral vectors, should continue to be explored so we can see the fulfillment of the promise of this form of treatment for patients with systemic and ocular conditions.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Ms. Leung is a medical writer at ORA Clinical Research and Development.
1. Pavan-Langston D. Viral Disease in the Cornea and External Eye. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. 2nd ed. Philadelphia: WB Saunders Co., 2000:846-893.
2. Marangon FB, Miller D, Alfonso E. Laboratory results in ocular viral diseases: Implications in clinical-laboratory correlation. Arq Bras Oftalmol 2007;70:2:189-94.
3. Bradshaw CS, Denham IM, Fairley CK. Characteristics of adenovirus associated urethritis. Sex Transm Infect 2002;78:6:445-7.
4. Schrauder A, Altmann D, Laude G, et al. Epidemic conjunctivitis in Germany, 2004. Euro Surveill 2006;11:7:185-7.
5. Zhao H, Granberg F, Pettersson U. How adenovirus strives to control cellular gene expression. Virology 2007;363:2:357-75.
6. Koehler DR, Martin B, Corey M, Palmer D, Ng P, Tanswell AK, Hu J. Readministration of helper-dependent adenovirus to mouse lung. Gene Ther 2006;13:9:773-80.
7. Yang L, Lochmuller H, Luo J, et al. Adenovirus-mediated dystrophin minigene transfer improves muscle strength in adult dystrophic (MDX) mice. Gene Ther 1998;5:3:369-79.
8. Li H, Alonso-Vanegas M, Colicos MA, Jung SS, Lochmuller H, Sadikot AF, Snipes GJ, Seth P, Karpati G, Nalbantoglu J. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin Cancer Res 1999;5:3:637-42.
9. Edamura K, Nasu Y, Takaishi M, Kobayashi T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T, Watanabe M, Huh NH, Kumon H. Adenovirus-mediated REIC/Dkk-3 gene transfer inhibits tumor growth and metastasis in an orthotopic prostate cancer model. Cancer Gene Ther 2007;14:9:765-72.
10. Butt AL, Chodosh J. Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea 2006;25:2:199-202.
11. Moshirfar M, Welling JD, Feiz V, et al. Infectious and noninfectious keratitis after laser in situ keratomileusis: Occurrence, management, and visual outcomes. J Cataract Refract Surg 2007;33:3:474.
12. Jawetz E. The story of shipyard eye. Br Med J 1959;1:5126:873-6.
13. Shipyard eye. Br Med J (Clin Res Ed) 1981;283:6292:629-30.
14. Asencio-Duran M, Romero-Martin R, Garcia-Martinez JR, et al. [Nosocomial outbreak of epidemic keratoconjunctivitis in a neonatal intensive care unit]. Arch Soc Esp Oftalmology 2007;
82:2:73-9.
15. Uchio E, Takeuchi S, Itoh N, et al. Clinical and epidemiological features of acute follicular conjunctivitis with special reference to that caused by herpes simplex virus type 1. Br J Ophthalmol 2000;84:9:968-72.
16. Hillenkamp J, Reinhard T, Ross RS, et al. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: Results of a controlled clinical pilot study. Ophthalmology 2002;109:5:845-50.
17. Wildner O, Hoffmann D, Jogler C, Uberla K. Comparison of HSV-1 thymidine kinase-dependent and -independent inhibition of replication-competent adenoviral vectors by a panel of drugs. Cancer Gene Ther 2003;10:10:791-802.
18. Trousdale MD, Dunkel EC, Nesburn AB. Effect of flurbiprofen on herpes simplex keratitis in a rabbit model. Invest Ophthalmol Vis Sci 1980;19:3:267-270.
19. Oshima Y, Sakamoto T, Hisatomi T, Tsutsumi C, Ueno H, Ishibashi T. Gene transfer of soluble TGF-beta type II receptor inhibits experimental proliferative vitreoretinopathy. Gene Ther 2002;9:18:1214.
20. Shen J, Taylor N, Duncan L, Kovesdi I, Bruder JT, Forrester JV, Dick AD. Ex vivo adenovirus mediated gene transfection of human conjunctival epithelium. Br J Ophthalmol 2001;85:7:861-7.
21. Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther 2006;13:3:528-37.
22. Woods NB, Bottero V, Schmidt M, et al. Gene therapy: Therapeutic gene causing lymphoma. Nature 2006;440:7088:1123.
23. Pike-Overzet K, van der Burg M, Wagemaker G, et al. New insights and unresolved issues regarding insertional mutagenesis in X-linked SCID gene therapy. Mol Ther 2007; Nov;15:11:1910-6.
24. Lamartina S, Cimino M, Roscilli G, Dammassa E, Lazzaro D, Rota R, Ciliberto G, Toniatti C. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: Stable gene transfer, regulated gene expression and therapeutic efficacy. J Gene Med 2007;9:10:862-74.
25. Surace EM, Auricchio A. Versatility of adeno-associated virus vectors for retinal gene transfer. Vision Res 2007.
26. Gong N, Pleyer U, Volk HD, Ritter T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene Ther 2007;14:6:484-90.
27. Hsiao CH, Sah WJ, Soya K, Ma DH, Im YW, Zhang F, Hwang DG. Effects of SV40 T antigen transduction on human corneal endothelial cell wound healing in vitro. J Ocul Pharmacol Ther 2005;21:5:353-66.
28. Austin BA, Halford WP, Williams BR, Carr DJ. Oligoadenylate synthetase/protein kinase R pathways and alphabeta TCR+ T cells are required for adenovirus vector: IFN-gamma inhibition of herpes simplex virus-1 in cornea. J Immunol 2007;178:8:5166-72.
29. Rolling F, Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Péréon Y, Cherel Y, Ali RR, Hamel C, Moullier P. Gene therapeutic prospects in early onset of severe retinal dystrophy: Restoration of vision in RPE65 Briard dogs using an adenovirus-associated vector serotype 4 vector that specifically targets the retinal pigmented epithelium. Bull Mem Acad R Med Belg 2006;161:10-12:497-508; discussion 508-9.



