 |
|
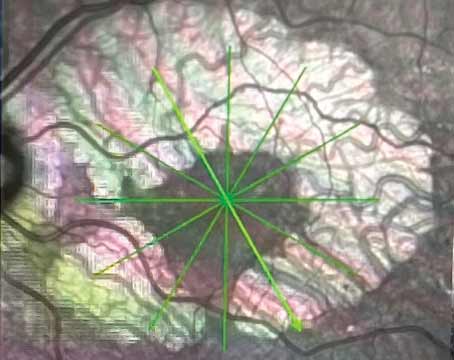
Figure 1. An eye with clinically significant macular edema. (All images courtesy of David Boyer, MD) |
The pipeline for diabetic macular edema treatments has expanded in recent years, with several promising products in various phases of clinical trials. The big news in DME treatment, however, is the FDA approval of Roche’s Vabysmo (faricimab-svoa) in late January.
Vabysmo targets and inhibits two disease pathways linked to a number of vision-threatening retinal conditions by neutralizing angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A). It’s the only FDA-approved injectable eye medication for neovascular age-related macular degeneration and DME that improves and maintains vision with treatment intervals up to four months apart in the first year following four initial monthly doses. Treatment intervals are based on the patient’s anatomy and vision outcomes.1
The approvals were based on positive results from four Phase III studies, which consistently showed that patients treated with Vabysmo given at intervals of up to four months achieved non-inferior vision gains versus aflibercept given every two months in the first year. Vabysmo was well-tolerated in all four studies, with conjunctival hemorrhage being the most common adverse reaction reported (7 percent).1
According to Chicago’s Jennifer I. Lim, MD, faricimab is going to make an impact on treatment. “It has an exceptionally long durability compared to other drugs,” she says. “The clinical trials are truly relevant for today’s retina specialist because they incorporate an adjustment of the dosing intervals based on disease activity after loading in the case of AMD, or a personalized treatment-interval arm in the case of the DME trials. So one can actually use the study to model how you will apply the treatment for your patients. I think the most exciting thing about faricimab is that more than 50 percent of patients can go about four months after loading. Also, more than 80 percent can go 12 weeks or more. That’s huge! Additionally, the OCT data show that the faricimab eyes had a better drying effect. In other words, there was less edema within the retina, and more eyes had complete resolution of fluid. That biomarker result explains the extended durability. I think that’s really exciting.”
While Vabysmo has achieved FDA approval, other promising treatments remain in the pipeline.
KSI-301 (Kodiak Sciences)
KSI-301 is an investigational anti-VEGF therapy built on Kodiak’s Antibody Biopolymer Conjugate Platform. It’s designed to maintain potent and effective drug levels in ocular tissues for longer than currently available agents.2 In early February, Kodiak completed enrollment in its GLEAM and GLIMMER Phase III clinical trials, which are global, multicenter, randomized studies designed to evaluate the efficacy, durability and safety of KSI-301 in patients with treatment-naïve DME. In each of these studies, patients are randomized to receive either intravitreal KSI-301 on an individualized dosing regimen every eight to 24 weeks after only three loading doses, or intravitreal aflibercept every eight weeks after five loading doses. Each study is expected to enroll approximately 450 patients worldwide; the primary endpoint is the change from baseline in best-corrected vision at a year. Patients will be treated and followed for two years.
“KSI-301 is a very-long-acting anti-VEGF,” explains the Cleveland Clinic’s Peter K. Kaiser, MD. “It lasted six months or longer in approximately two-thirds of patients in its Phase Ib study, and diabetic patients were able to go even longer. It still requires intravitreal injections, of course, and they’re testing diabetic retinopathy and DME at the same time.”
Thomas W. Stone, MD, who is in practice in Lexington, Kentucky, adds, “Through our studies, we feel there is potential with this molecule. It’s an anti-VEGF, and physically it’s a thicker liquid, like cold maple syrup or molasses. Patients tolerate the injections well, and it’s probably the next anticipated agent that’s going to find a place on your shelf.”
Brolucizumab
Brolucizumab (Beovu) (Novartis) is a VEGF-A inhibitor. In its Phase III trials (KESTREL and KITE), brolucizumab 6 mg showed robust visual gains and anatomical improvements with an overall favorable benefit/risk profile in patients with DME, compared with aflibercept.3
These studies were double-masked, 100-week, multicenter, active-controlled, randomized trials. Study participants were randomized 1:1:1 to brolucizumab 3 mg/6 mg or aflibercept 2 mg in KESTREL (n=566) or 1:1 to brolucizumab 6 mg or aflibercept 2 mg in KITE (n=360). Patients in the brolucizumab groups received five loading doses every six weeks followed by dosing every 12 weeks, with optional adjustment to every eight weeks if disease activity was identified. The primary endpoint was best-corrected visual acuity (BCVA) change from baseline at Week 52.
 |
|
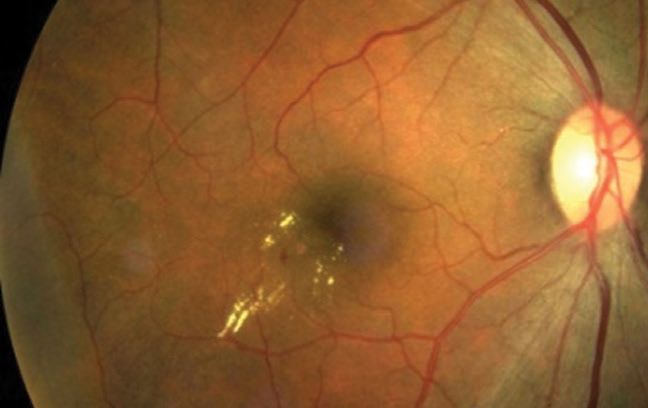
Figure 2. OCT of the eye from Figure 1 showing thickening of the retina. |
At Week 52, brolucizumab 6 mg was found to be noninferior to aflibercept in mean change in BCVA from baseline. More subjects achieved central subfield thickness less than 280 µm, and fewer had persisting subretinal and/or intraretinal fluid versus aflibercept. More than half of brolucizumab 6 mg subjects were maintained on every 12 weeks dosing after loading. In KITE, brolucizumab 6 mg showed superior improvements in change of central subfield thickness from baseline from week 40 to week 52 versus aflibercept. The incidence of serious ocular adverse events was 3.7 percent for brolucizumab 3 mg, 1.1 percent for brolucizumab 6 mg, and 2.1 percent for aflibercept in KESTREL; it was 2.2 percent for brolucizumab 6 mg and 1.7 percent for aflibercept in KITE.
“Beovu is a great drug for drying up the retina, and that’s been shown in clinical trials for age-related macular edema and DME,” says Dr. Lim. “The clinical trials did show the inflammatory responses are less likely to be seen in diabetes than in AMD. However, because we’ve seen that it can cause a severe retinal vasculitis, I personally am not willing to take the risk.”
Dr. Stone agrees. “Beovu has had problems with safety,” he says. “In the nAMD trials, more than 4 percent of patients experienced some form of inflammation with the shot, with a smaller subset of less than 1 percent of patients experiencing occlusive vasculitis with permanent vision loss. So, when it first came out, we were using it. Then, when we saw safety issues, everybody pulled back. Now, we’re only using it if we don’t have another option.”
Susvimo (Genentech)
The FDA recently approved Genentech’s Susvimo, formerly known as the port delivery system (PDS) with ranibizumab, for the treatment of patients with wet AMD who have previously responded to at least two anti-VEGF injections.
It continuously provides 100 mg/mL ranibizumab injections intravtreally via an ocular implant, which is surgically inserted into the eye during a one-time, outpatient procedure and then is refilled every six months.
“The PDS is currently in studies for diabetes,” Dr. Lim says. “In the future, it may be another option for people who need frequent injections, or for proliferative retinopathy that requires anti-VEGF, not just macular edema from diabetes.”
GB-102 (Graybug Vision)
GB-102 is a VEGF receptor-1 agonist that’s currently in Phase II clinical trials. The company conducted an open-label, single-injection trial in patients with macular edema, with a primary endpoint of the safety and tolerability of two dose levels of GB-102 (1 and 2 mg with optimized formulation).4 The study included six centers in the United States that enrolled 21 patients who had received at least three prior injections of anti-VEGF and who had at least some response within the past 24 months.
At enrollment, patients required, on average, eight injections per year to control their disease. Eligible patients received GB-102 (1 or 2 mg) at day one and were followed monthly.
There were no drug-related non-ocular adverse events in the trial. The 2-mg dose was associated with medication present in the anterior chamber in five of 11 patients. Most adverse events occurred in these patients, including two serious adverse events in a single patient. It’s believed that too many microparticles were injected in the 2-mg dose (approximately 2 million) to allow adequate aggregation. As a result, the company has discontinued the development of the 2-mg dose.4
The GB-102 1-mg dose met its primary endpoint of safety and tolerability, with seven of 10 patients demonstrating no adverse events. None of the patients had inflammation, and all adverse events were mild to moderate and resolved without long-term consequences. The microspheres aggregated well in the 1-mg dose and formed a depot at the bottom of the eye. As the depot eluted drug, the size decreased, and at six months there was only a small amount of the depot left.
ADVM-022 (Adverum)
ADVM-022 is a VEGF-A inhibitor in Phase II clinical trials. It’s an adeno-associated viral vector encoding aflibercept that’s been optimized for intravitreal delivery and strong protein expression.
Long-term expression and efficacy of ADVM-022-derived aflibercept were evaluated in a laser-induced choroidal neovascularization model in non-human primates.5 Ocular safety was evaluated, following long-term suppression of VEGF, by clinical scoring (inflammatory parameters) as well as OCT and electroretinography. Intravitreal administration of ADVM-022 was well-tolerated and resulted in sustained aflibercept levels in ocular tissues. Additionally, administration of ADVM-022 13 months before laser-induced choroidal neovascularization prevented the occurrence of clinically relevant CNV lesions, to the same degree as a bolus of aflibercept given at the time of the laser. These results demonstrate that a single intravitreal administration of ADVM-022 may provide a safe and effective long-term treatment option for DME and may ultimately improve patients’ visual outcomes.
Clinical trials are currently under way to evaluate the safety and efficacy of a single intravitreal injection of ADVM-022.
KVD001 (KalVista)
KVD001 is an intravitreally administered plasma kallikrein inhibitor that’s completed a Phase II clinical trial in patients with DME.6
The clinical trial evaluated the safety and efficacy of KVD001 in patients who have received previous anti-VEGF therapy, yet continue to experience reduced visual acuity and significant edema. The primary efficacy endpoint in the trial was change in best-corrected visual acuity at 16 weeks compared to sham. The 6-µg dose showed a difference of +2.6 letters versus sham, which wasn’t statistically significant, and the 3-µg dose showed a difference of +1.5 letters. No significant differences were observed in the secondary endpoints of central subfield thickness or the diabetic retinopathy severity scale. KVD001 was safe and well-tolerated with no drug-related serious adverse events.
The Rest of the Field
According to Dr. Lim, gene therapy is further down the pipeline. “I’m a little bit cautious about gene therapy, not just because some inflammatory reactions can develop, but primarily because you can’t shut it down,” she says. “Once you put an ‘anti-VEGF factory’ genetically into the eye, it’s going to keep producing anti-VEGF, and we know that we need some VEGF for viability of tissues. So, until we learn how to modulate the ‘factory’ and learn more about the long-term outcomes, I’m less excited about that for diabetic applications.”
David S. Boyer, MD, in practice in Los Angeles, believes that a multifactorial approach may be best. “You have longer-acting steroids that may be able to last six months,” he notes. “We know steroids work, but they have a number of potential risks. Pills are interesting because if you have a pill that will reduce the diabetic retinopathy severity, it may also play a role systemically in kidney function or even neuropathy. So, I think, if you could take a pill rather than getting a shot, there may be greater acceptance. Several oral medications attacking different pathways (REF-1, connexin-43 channels, CCR5 eotaxin inhibitor, AOC3, CB receptor or the kallikrein pathway) are under development.”
He adds that drops are being studied, but drops are exceedingly difficult to get to the back of the eye in a concentration sufficient to cause a marked improvement on a consistent basis. “They do work, but they’re inconsistent, in that only 30 percent to 50 percent of patients improve,” he says. “There have been several improvements in the delivery system of drops that may allow us to use drops in the future. Treatment comes down to several things: trying to reduce the treatment burden of the VEGFs; trying to reduce the number of patients who don’t respond to therapy at the present time; and trying to treat earlier in the course of the disease to prevent vision-threatening complications. The earlier we can treat, the better chance we have of people keeping their vision. So, I think it’s a multifactorial approach. Some are treating the macular edema, and some are treating to reduce the diabetic retinopathy severity so we can go earlier and possibly prolong or reduce the chances of the patient progressing.”
Dr. Boyer has a financial interest in Adverum Biotechnologies, Genentech, Graybug Vision, Roche and Novartis. Dr. Kaiser is a consultant to Kodiak, Oxurion, Regeneron, Novartis, RegenxBio, Ocuphire and Allergan. Dr. Lim is a consultant to Genentech and Novartis. She has previously done research for or received grants from Genentech, Roche and Graybug. Dr. Stone is an investigator for the Kodiak and Genentech trials.
1. Genentech/Roche update. https://www.roche.com/investors/updates/inv-update-2022-01-31.htm. Accessed February 18, 2022.
2. Kodiak company information. https://kodiak.com/our-pipeline/. Accessed February 18, 2022.
3. Brown DM, Emanuelli A, Bandello F, et al. KESTREL and KITE: 52-week results from two phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol. 2022 Jan 13: Online ahead of print.
4. Graybug pipeline report. https://www.graybug.vision/our-technologies-and-pipeline/#gb102. Accessed February 18, 2022.
5. Gelfman CM, Grishanin R, Bender KO, et al. Comprehensive preclinical assessment of ADVM-022, an intravitreal anti-VEGF gene therapy for the treatment of neovascular AMD and diabetic macular edema. J Ocul Pharmacol Ther. 2021;37:3:181-190.
6. Kalvista pipeline report. https://www.kalvista.com/products-pipeline/kalvista-dme. Accessed February 18, 2022.