Nowadays, most glaucoma treatment and management decisions are based on OCT, RNFL and visual field data, with artificial intelligence expected to supplement our progression detection capabilities in the future. In addition to these imaging modalities and future algorithms, checking for the presence of optic disc hemorrhage can provide information about a patient’s disease state and likelihood of progression.
Several studies, including the Ocular Hypertension Treatment Study,1 the Early Manifest Glaucoma Trial,2 the Collaborative Normal-Tension Glaucoma Study3 and others4 confirm that patients with disc hemorrhages are at high risk for progression. It’s important to examine patients’ optic nerves, even if the patient has just come in for a quick pressure check.
Disc hemorrhage is identified on examination of the optic nerve and/or on color fundus photographs. The OCT RNFL image doesn’t identify disc hemorrhage. It may take time—as much as a year or more—to identify changes in the visual field or OCT RNFL due to disc hemorrhage.5
Here, I’ll discuss how to proceed with a patient who has an optic disc hemorrhage.
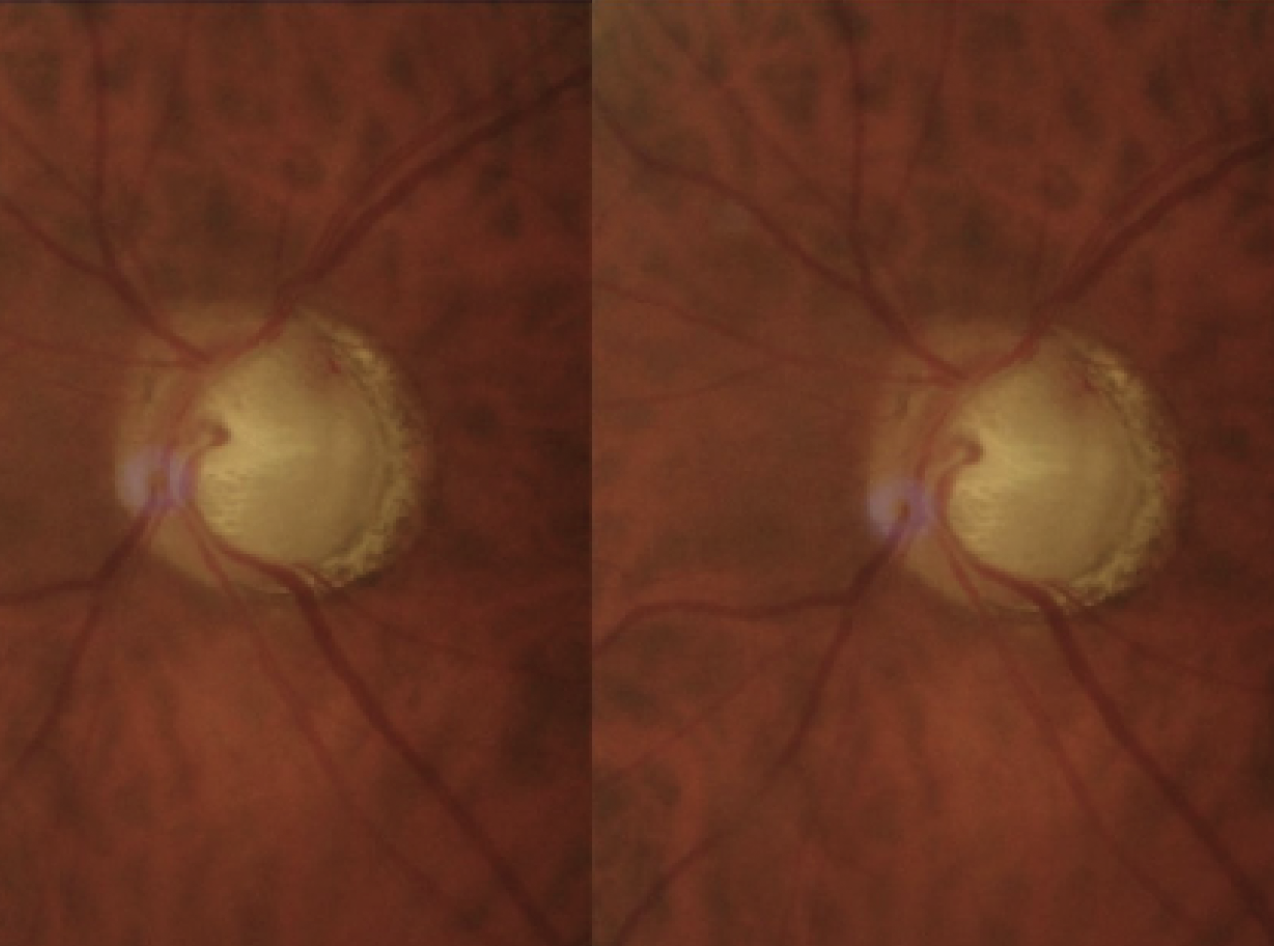 |
| Figure 1. A disc hemorrhage in resorption (Case #1). |
What To Do
Optic disc hemorrhages are transitory and not every patient with a disc hemorrhage will progress, but it’s an important warning sign that shouldn’t be missed. The blood itself isn’t as concerning as the underlying cause. When a patient presents with a disc hemorrhage, there are three things we must do:
1. Check if the IOP is within target range and assess fluctuation and compliance. Is the IOP consistently controlled? The pressures we measure in the office might not capture fluctuations in pressure that happen at other times of day and night, and it’s not uncommon for patients to have poor compliance with their medications.
If one has access to at-home pressure monitoring tools such as the Triggerfish contact lens or iCare Home tonometer, these can provide a general idea of how stable the pressures are. One approach I like is checking the patient’s intraocular pressure in a supine position. When patients are supine, their pressures are usually higher.6
2. Follow the area carefully. After a disc hemorrhage, monitor patients using alternating 10-2 and 24-2 visual fields and RNFL/retinal ganglion cell OCT. Follow the patient every three months or so and watch the areas where the disc hemorrhage occurred to ensure the patient isn’t going to develop further progression in that area. It’s key to use the 10-2 visual field to avoid missing early defects or small changes in the central visual field. If vision is poor, a size-five stimulus must be used.
3. Rule out other causes. Confirm there are no other IOP-independent risk factors for progression such as nocturnal hypotension or sleep apnea.7,8
Case #1
A patient with severe normal-tension glaucoma with pressures in the mid-teens started to progress after a disc hemorrhage in 2017 (Figure 1). He had previously undergone selective laser trabeculoplasty and used travoprost every night at bedtime, timolol 0.5% every day upon awakening in the morning and brinzolamide twice daily. He was allergic to brimonidine.
The patient was followed closely using 10-2 visual fields to confirm and document progression (Figure 2). Deep sclerectomy was performed to further lower the pressure to safer levels.
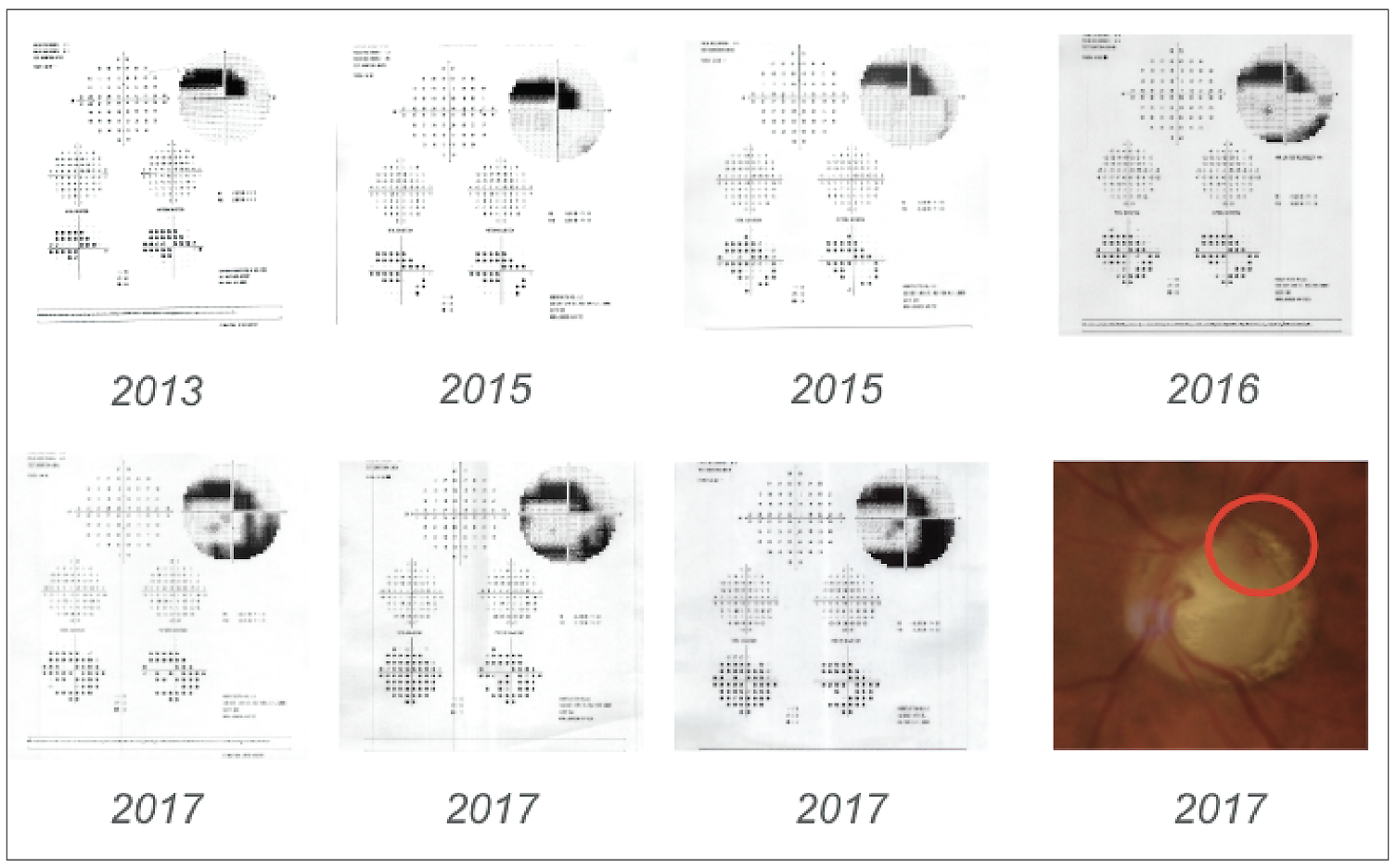 |
| Figure 2. After a disc hemorrhage in 2017, the patient in Case #1 began to progress, demonstrating an arcuate defect on 10-2 visual fields. Pressures were stabilized following surgical intervention. |
Case #2
This 83-year-old patient with severe pseudoexfoliation glaucoma and previous trabeculectomy began to develop disc hemorrhages in the same area in 2018. Between 2018 and 2020, we detected five disc hemorrhages superotemporally (Figure 3). Corresponding inferonasal spots started to appear in 10-2 visual fields.
Surgery was suggested, but the patient was hesitant to proceed as she was concerned about the risks associated to surgery. Based on careful monitoring, we determined that the progression was slow. Since progression was slow and she was concerned about possible surgical complications, we decided to maximize her medical treatment to further decrease intraocular pressures. So far, her glaucoma has been fairly stable, as demonstrated by the downward-trending IOP from the GPA analysis and by the RGC OCT, which is very useful for analyzing progression in severe glaucoma.
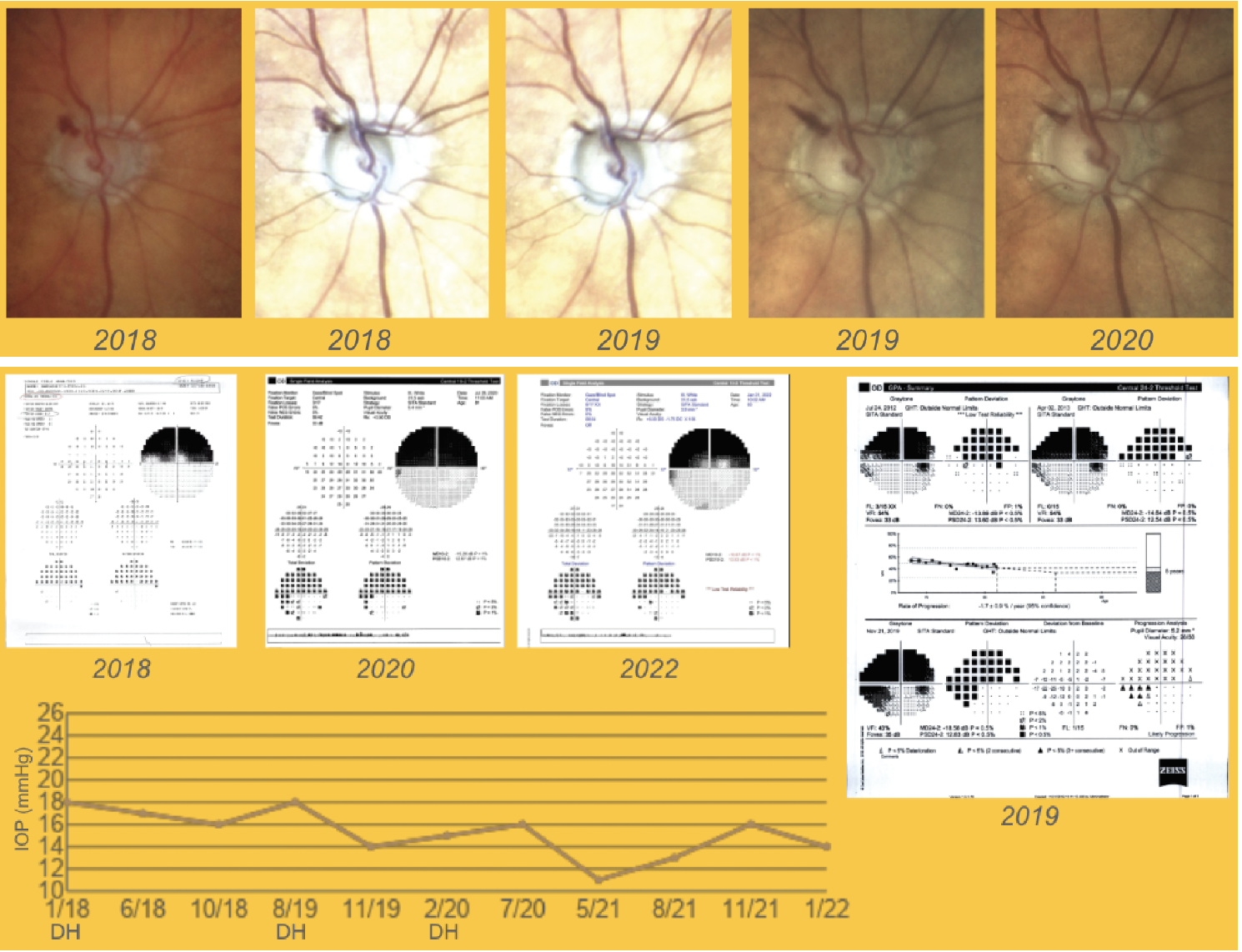 |
| Figure 3. A patient in her 80s developed several disc hemorrhages over a two-year period in the superotemporal area (Case #2). Surgery was recommended, but the patient preferred to use maximal medical therapy, which was adjusted to further decrease IOP, and has remained stable. Some inferonasal spots were seen on the 2020 10-2 visual fields but IOP remained overall stable. Click image to enlarge. |
It's Not Always Glaucoma
Most of the time, disc hemorrhages in patients with glaucoma are related to the disease itself. These glaucomatous disc hemorrhages are flame-shaped and located adjacent to an area of a retinal nerve fiber layer defect. However, when the disc hemorrhage isn’t flame-shaped, is broader or not related to a specific RNFL defect, we have to consider other causes.
Other causes of disc hemorrhage may include retinal diseases, ocular trauma, posterior vitreous detachment, and brain or optic nerve diseases; and systemic diseases such as hypertension, diabetes mellitus, hematologic disorders, migraine and systemic medications such as blood thinners.9
Most of the time, asking patients questions and using clinical data will help us make the diagnosis. For example, a posterior vitreous detachment is a common cause and patients will often say they’ve noticed a new floater. In patients with uncontrolled diabetes and diabetic retinopathy, we may see disc hemorrhages that are associated with other retinal findings. Blood discoloration may suggest coagulation problems, and migraines may lead to disc hemorrhage due to vasospasm.
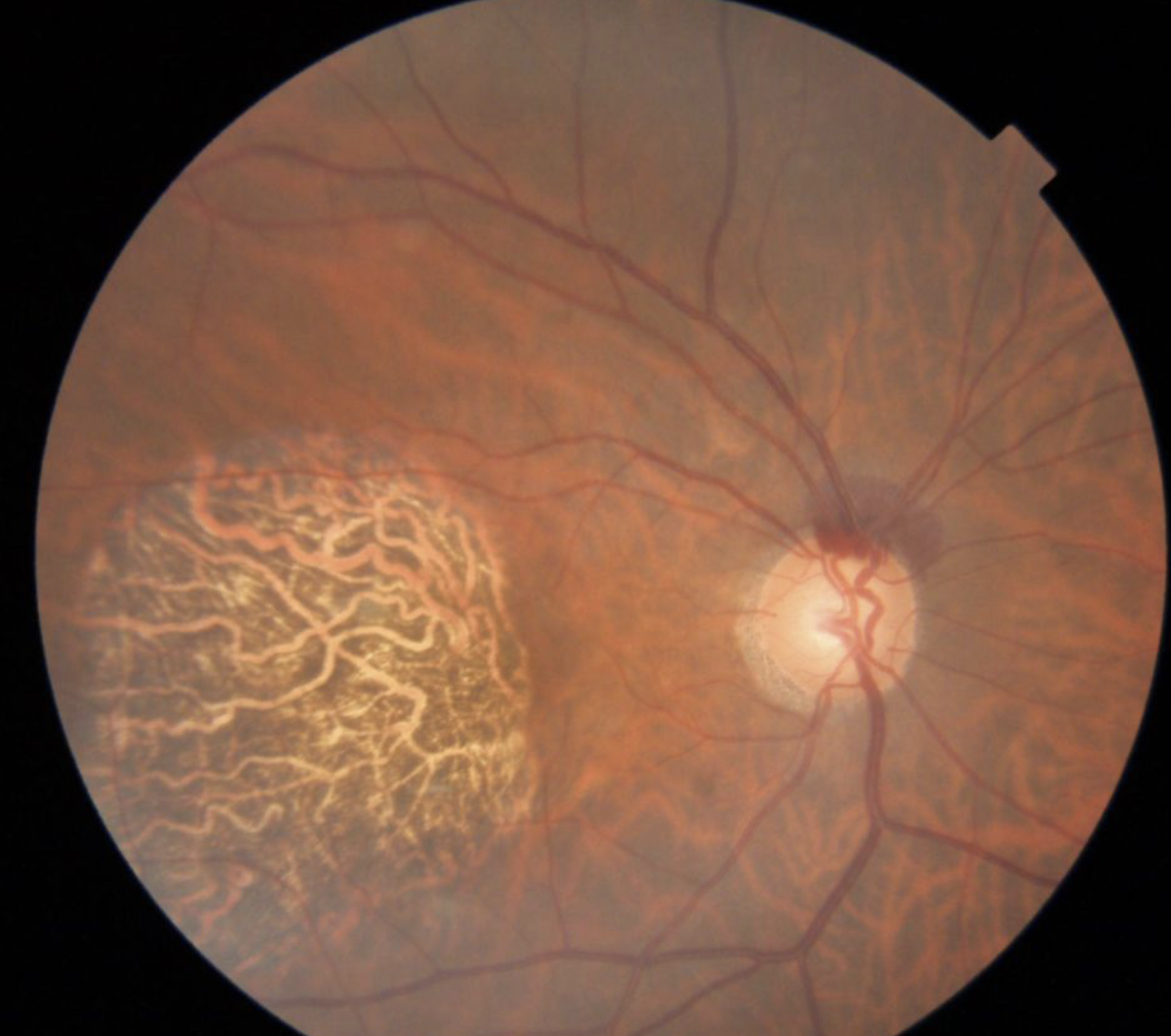 |
| Figure 4. A broad, superior, non-glaucomatous disc hemorrhage. Glaucomatous disc hemorrhages are usually flame-shaped and located next to a retinal nerve fiber layer defect. |
Figure 4 shows an example of a broad superior disc hemorrhage that’s not related to glaucoma. In this example, the patient was vitrectomized (so we ruled out PVD), asymptomatic and being followed for ocular hypertension. He was also using blood thinners. We concluded that blood-thinner use was the cause, which put him at a higher risk for disc hemorrhages or any retinal hemorrhages.
It’s very important to perform a dilated fundus exam to rule out retinal causes such as branch retinal vein occlusions or central vein occlusions. Vein occlusions may cause more dramatic disc hemorrhages that are spread widely across the retina, unlike glaucomatous ones (Figure 5).
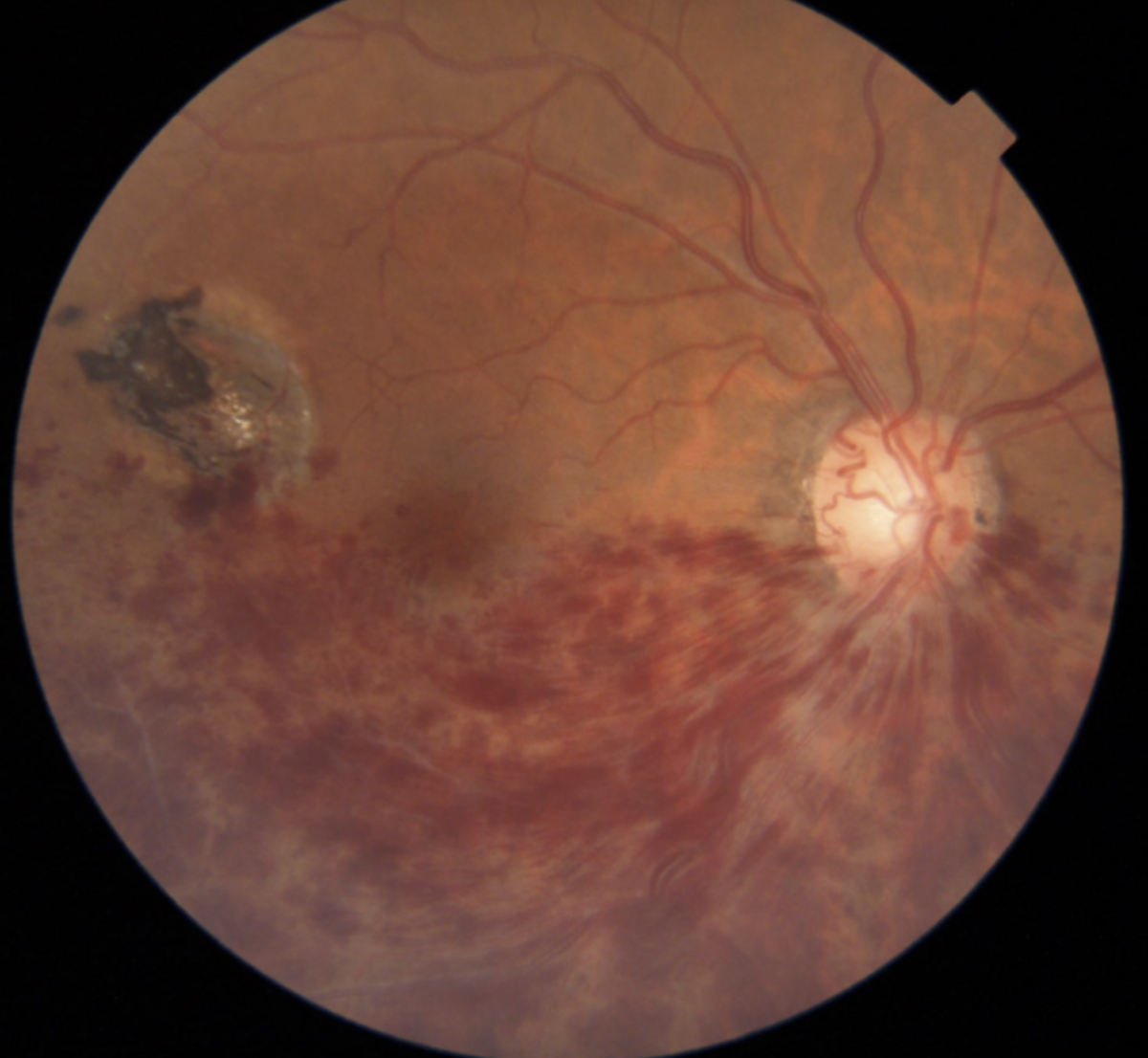 |
| Figure 5. A branch retinal vein occlusion results in a more dramatic, widespread hemorrhage. |
Normal Tension Glaucoma
Be vigilant in normal-tension glaucoma patients. These patients present more often with disc hemorrhages than patients with other glaucoma subtypes. It may be that these hemorrhages aren’t solely pressure-related but could result from mechanical forces or vascular dysregulation. We know that normal tension glaucoma patients have a higher frequency of vascular dysregulation phenomenon,10 and that it’s more common among women, sometimes with a previous history of migraines or Raynaud’s phenomena—both of which are also signs of vascular dysregulation.
In summary, optic disc hemorrhages associated with glaucoma are important warning signs of possible disease progression. Perform a thorough assessment, monitor the patient closely for progression and adjust treatment accordingly.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis.
Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
Dr. Silva is in private practice at Gentile Retina in New York City, and is affiliated to NYU as a clinical instructor in the Department of Ophthalmology at NYU Grossman School of Medicine.
1. Budenz DL, Anderson DR, Feuer WJ, Beiser JA, Schiffman J, Parrish II RK, Piltz-Seymour JR, Gordon MO, Kass MA, et al. Ocular Hypertension Treatment Study Group. Detection and prognostic significance of optic disc hemorrhages during the OHTS. Ophthalmology 2006;113:12:2137-43
2. Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: The EMGT. Arch Ophthalmol 2003;121:1:48-56.
3. Drance S, Anderson DR, Schulzer M, et al. Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in NTG. Am J Ophthalmol 2001;131:6:699-708.
4. Ishida K, Yamamoto T, Sugiyama K, and Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma.
5. Siegner SW, Netland PA. Optic disc hemorrhages and progression of glaucoma. Ophthalmology 1993;103:10141024.
6. Borrone R. A new strategy for diurnal intraocular pressure curve. Eur J Ophthalmol 2012;22:6:964-71.
7. Charlson ME, Gustavo de Moraes C, Link A, Wells MT, Harmon G, Peterson JC, Ritch R, Liebmann J. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology 2014;121:10:2004-12.
8. Faridi O, Park S, Liebmann JM, Ritch R. Glaucoma and OSAS. Clin Exp Ophthalmol 2012;40:4:408-19.
9. Reza Razeghinejad M, Hossein Nowroozadeh M. Optic disk hemorrhage in health and disease. Major review. Surv Ophthalmol 2017;62:784-802.
10. Pasquale LR. Vascular and autonomic dysregulation in primary open-angle glaucoma. Curr Opin Ophthalmol 2016;27:2:94-101.



