Presentation
A 78-year-old male first noticed a growth on the surface of his left eye in the fall of 2022, which increased in size over several months and began to cause blurred vision. He presented to his primary ophthalmologist in May 2023 for an annual exam. His ophthalmologist noted a concerning ocular surface mass and referred the patient to Wills Eye Hospital for a consultation with a cornea specialist.
History
The patient had a history of type 2 diabetes and moderate to severe non-proliferative diabetic retinopathy for which he was followed yearly by his general ophthalmologist. He had a known stable choroidal nevus in the right eye which was monitored with annual dilated exams and fundus photos. His surgical history was notable for cataract extraction with intraocular lens implantation in both eyes seven years previously.
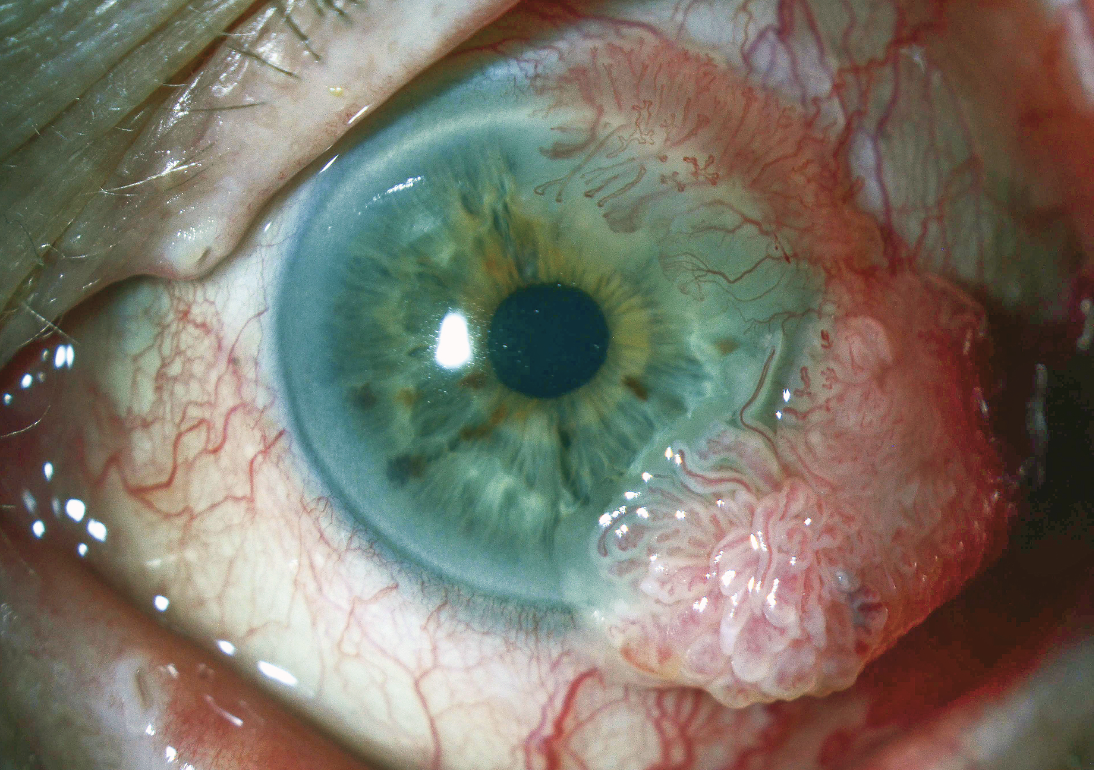 |
| Figure 1. A limbal mass composed of multiple fronds of epithelium with vascular cores. Feeder vessels are present. |
Examination
At presentation, best-corrected visual acuity was 20/25 in his right eye and 20/100 in his left eye. His pupils were round and reactive in both eyes without an afferent pupillary defect in either eye. Intraocular pressures were 16 mmHg in the right eye and 15 mmHg in the left eye. Extraocular motility and confrontation visual fields were full bilaterally.
Anterior segment examination of the left eye disclosed a papillomatous limbal lesion located between 12 and 6 o’clock. The lesion extended about 2.5 mm onto the cornea. Between 12 and 3 o’clock, it was relatively flat. Between 3 and 6 o’clock, it was elevated, with large feeder vessels (Figure 1). Anterior segment optical coherence tomography revealed an abrupt transition between the healthy and the thickened diseased epithelium (Figure 2).
Other notable findings included mild meibomian gland dysfunction bilaterally and posterior chamber intraocular lenses in good position in both eyes. Eversion of both upper eyelids revealed no significant findings. The posterior segments of the right eye and the left eye were unremarkable.
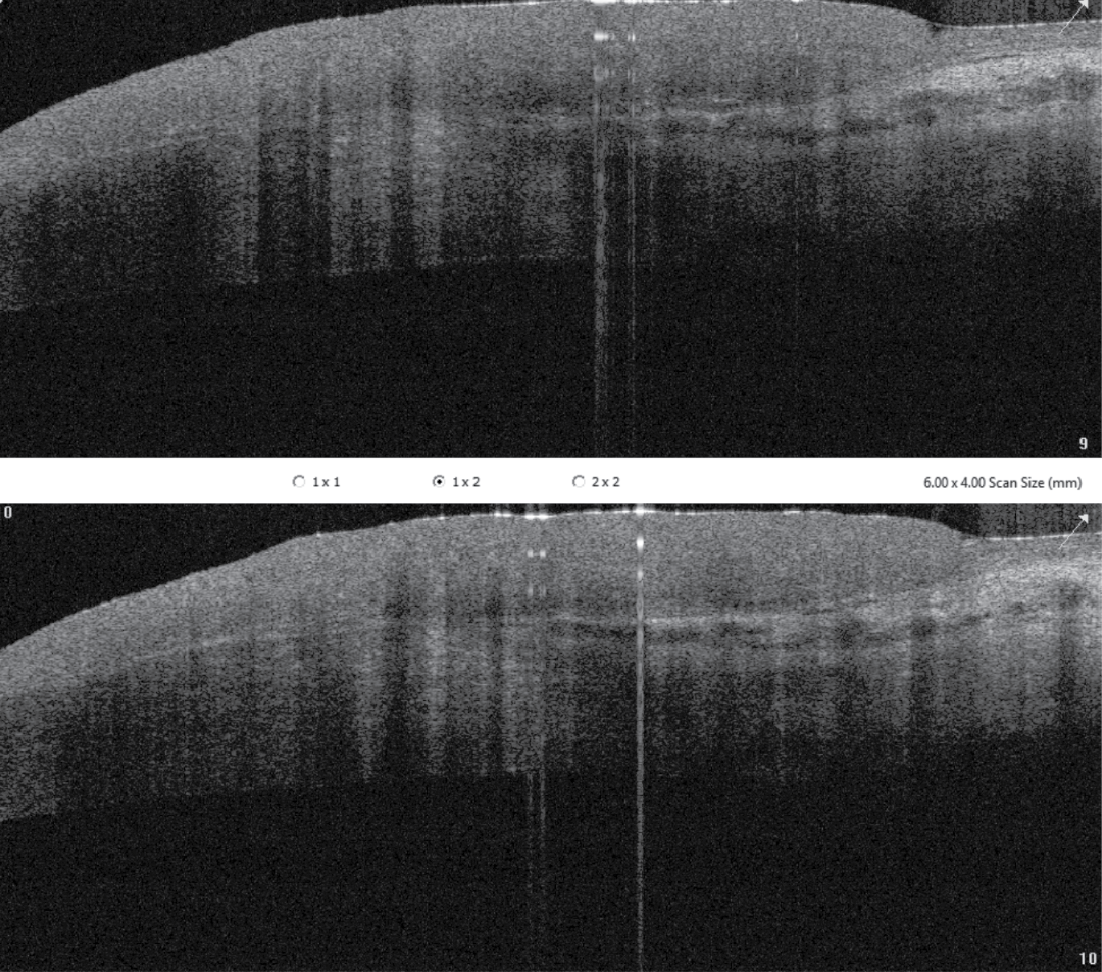 |
| Figure 2. AS-OCT of the limbal mass photographed in Figure 1. |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
The conjunctival mass was excised with cryotherapy of the limbus and margins, followed by amniotic membrane transplantation (AMT) in June 2023. Histopathology disclosed a papillomatous lesion composed of fronds of thickened dysplastic epithelium surrounding cores of inflamed fibrovascular tissue (Figure 3). The entire thickness of the epithelium was replaced by severely dysplastic epithelial cells, consistent with a diagnosis of papillary squamous cell carcinoma in situ. Severely dysplastic epithelium was present at the limbal and corneal tissue margins.
Because the lesion was large and was incompletely excised, the patient was treated postoperatively with two cycles of adjuvant topical 5-fluorouracil (four times daily for a week, with three weeks off). Six weeks postoperatively, there was no evidence of recurrence.
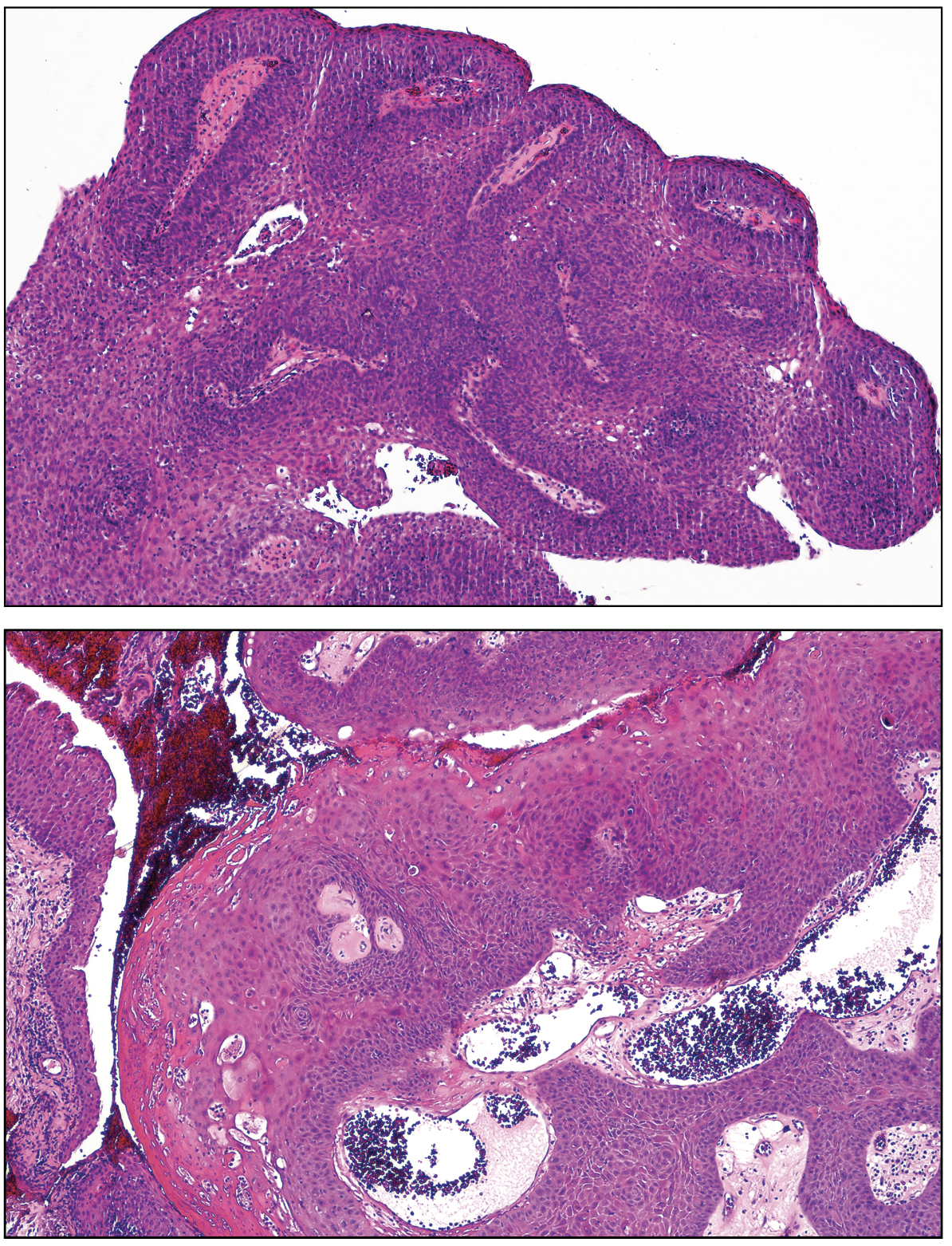 |
| Figure 3. Epithelium is totally replaced by atypical squamous cells consistent with squamous cell carcinoma in situ. Hematoxyin & eosin. Top x25, lower x100. |
Discussion
The term ocular surface squamous neoplasia (OSSN) refers to a neoplastic spectrum that involves the squamous cells of the conjunctival and corneal epithelium. Most of the disease entities included in OSSN are rare, with incidences ranging from 0.1 to 35 cases per 1,000,000 people.1 Risk factors for developing OSSN include ultraviolet light and/or sun exposure, vitamin A deficiency, xeroderma pigmentosum, human immunodeficiency virus (HIV) and human papilloma virus (HPV).2-4 There is also a higher incidence among Caucasian populations and individuals with lighter colored irides.2,4
OSSN includes conjunctival intraepithelial neoplasia (CIN), corneal epithelial dysmaturation and dysplasia, and in situ and invasive squamous cell carcinoma (SCC).5 OSSN encompasses histologic features ranging from CIN to SCC. These features include varying degrees of epithelial dysplasia, such as CIN, which can progress to SCC in situ neoplasia, and finally invade through the epithelial basement membrane into the substantia propria as invasive SCC.6 Papillary SCC, as diagnosed in this patient, is a variant of SCC of the ocular surface. It’s diagnosed via clinical features as well as excisional biopsy and histopathological examination. This entity typically presents clinically as a unilateral mass at the intrapalpebral limbus that is vascularized and elevated, creating a papillary variant of OSSN. The mass can present with tortuous feeder vessels as well, as in this patient.6
AS-OCT, which was obtained for this patient, can be a helpful in vivo tool to assess and diagnose an ocular surface mass. A characteristic AS-OCT feature of OSSN is an abrupt transition between normal and neoplastic tissue, as shown in this case. AS-OCT also can demonstrate a thickened and hyperreflective epithelium and be helpful in evaluating for invasion. It can be helpful in cases where the clinical diagnosis isn’t abundantly clear, and also can help to assess progress after treatment.7
Excisional biopsy is a common treatment modality as it provides immediate resolution and allows for direct histopathological examination of the neoplastic tissue, although biopsy isn’t critical for a diagnosis of OSSN.8 Topical treatment modalities for OSSN include interferon-alpha2b, 5-fluorouracil, and/or mitomycin C. Topical therapy can be used to treat the entire ocular surface, which can be favorable in larger tumors, and can be used to avoid surgical complications. However, topical modalities require patient compliance with several months of therapy.9
Recurrence rates of OSSN range between 24 percent and 50 percent in the literature for surgical therapy alone,10 with recurrence rates reported higher at 56 to 69 percent when there are positive surgical margins.11 Topical pharmacotherapy has had lower reported recurrence rates ranging from 5 to 11 percent, although follow-up data may be limited.12 A meta-analysis demonstrated similar recurrence rates among topical therapy and surgical excision.12 Recurrence typically occurs within two years after surgery, and is highly dependent upon the surgical margins and use of adjuvant therapy. Cryotherapy is an important step of surgical management, as was performed in this patient. This treatment, when used immediately following tumor excision, causes thermal destruction of tumor cells and their vasculature, and can target residual tumor cells and therefore prevent recurrence.9 Recurrence rates of combined surgical excision and cryotherapy have been reported as low as 0 to 12.3 percent.13
This patient opted for surgical excision for immediate resolution, and we decided to initiate adjuvant topical therapy given the size of the lesion as well as positive tissue margins at both the corneal surface and the conjunctival portion of the tumor. This treatment addresses residual tumor cells and may reduce the risk of recurrence. AMT aided in the reconstruction of the ocular surface after the tumor was resected.
In conclusion, OSSN and particularly SCC can present with characteristic growths on the ocular surface with associated feeder vessels. AS-OCT and histopathologic examination are critical tools in the diagnosis of this entity. There are several treatment options available, including surgical and topical therapies. Ultimately, the treatment approach should be tailored to each patient’s individual goals of care.
1. Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology 2004;111:1747–1754.
2. Napora C, Cohen EJ, Genvert GI, Presson AC, Arentsen JJ, Eagle RC, Laibson PR. Factors associated with conjunctival intraepithelial neoplasia: A case control study. Ophthalmic Surg 1990;21:27–30.
3. Porges Y, Groisman GM. Prevalence of HIV with conjunctival squamous cell neoplasia in an African provincial hospital. Cornea 2003;22:1–4.
4. Scott IU, Karp CL, Nuovo GJ. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology 2002;109:542–547.
5. Basti S, Macsai MS. Ocular surface squamous neoplasia: A review. Cornea 2003;22:687–704.
6. Shields CL, Chien JL, Surakiatchanukul T, Sioufi, K, Lally, SE, Shields, JA. Conjunctival tumors: Review of clinical features, risks, biomarkers, and outcomes—The 2017 J. Donald M. Gass Lecture. Asia-Pacific Journal of Ophthalmology 2017;6:2:109-120.
7. Stevens SM, Reyes-Capo DP, Patel U, Choudhary A, Khzam RA, Tang V, Galor A, Karp CL, Dubovy S. Clinical and optical coherence tomography comparison between ocular surface squamous neoplasia and squamous metaplasia. Cornea 2023;42:4:429-434.
8. Polski A, Sibug Saber M, Kim JW, Berry JL. Extending far and wide: The role of biopsy and staging in the management of ocular surface squamous neoplasia. Clin Exp Ophthalmol 2019;47:2:193-200.
9. Hӧllhumer R, Williams S, Michelow P. Ocular surface squamous neoplasia: Management and outcomes. Eye (Lond) 2021;35:6:1562-1573.
10. Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: Analysis of 60 cases. Br. J. Ophthalmol 1999;83:98–103.
11. Tabin G, Levin S, Snibson G, et al. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology 1997;104:485-492.
12. Kozma K, Dömötör ZR, Csutak A, Szabó L, Hegyi P, Erőss B, Helyes Z, Molnár Z, Dembrovszky F, Szalai E. Topical pharmacotherapy for ocular surface squamous neoplasia: Systematic review and meta-analysis. Sci Rep 2022;12:1:14221.
13. Peksayar G, Altan-Yaycioglu R, Onal S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye 2003;17:228–232.