Organ transplantation has evolved significantly in recent years with the advent of several potent immunomodulatory agents. Organs such as bone marrow, kidney, heart, liver, lung, pancreas and intestine are transplanted with greater success than ever before. The ocular complications of organ transplantation are common. They are typically due to the underlying disease process, long-term immunosuppression and conditioning regimen. Anterior segment complications include keratoconjunctivitis sicca, pseudomembranous conjunctivitis, conjunctival graft-versus-host disease, bacterial and viral conjunctivitis, superficial punctate keratitis, sterile and infectious corneal ulceration and cataracts.e1,2 Posterior segment complications are less frequently observed, but they can have significant visual implications. In one report, 12.8 percent of 397 patients developed posterior segment complications after bone marrow transplantation (BMT).2 In general, retinal complications of organ transplantation may be divided into one of three types: infectious, microvascular and hematologic complications.
Infectious Complications
Infectious complications of organ transplantation account for the majority of mortality in transplant recipients. Fortunately, ocular infection is relatively uncommon. In one study, 2 percent of patients with BMT developed posterior segment infections.2 Because of the potentially fatal nature, infections are treated aggressively. The development of potent antibiotics has significantly reduced the problems of both systemic and ocular infections in these patients.
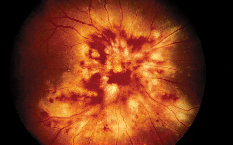 |
| Figure 1. A patient with CMV optic neuritis and retinitis, occurring 18 months following bone marrow transplantation. The patient was treated with intravenous foscarnet and ganciclovir therapy. |
Viral retinitis may be observed after organ transplantation. A common cause is cytomegalovirus (CMV) (See Figure 1). Approximately 1-2 percent of organ transplant recipients develop CMV retinitis.3 However, in the pediatric organ transplant population, the prevalence of CMV retinitis may be as high as 14 percent.4 The visual consequence from CMV retinitis can be significant, resulting in severe visual loss. The infection appears to be more frequently observed in patients with HLA-matched unrelated donor transplants than in patients with HLA-matched related sibling transplants.5 The use of certain immunomodulatory drugs such as mycophenolate mofetil has been linked to an increased risk of systemic CMV infection in organ transplantation, but it is uncertain if this holds true for ocular CMV infection.6 The infection usually responds well to antiviral agents such as systemic or intravitreal ganciclovir (injection or implant) and foscarnet. In addition, the prophylactic use of valacyclovir for CMV infection in organ transplantation has been suggested.
Valacyclovir has been shown to be efficacious and cost-effective as a CMV prophylaxis in renal, heart, and bone marrow transplantations.7
Viral retinitis can also result from herpes zoster. Varicella zoster virus infection such as shingles is a frequent complication after organ transplantation, but it is an uncommon cause of retinal complication. The clinical manifestations of herpes zoster retinitis are analogous to those of acute retinal necrosis, and the virus has also been linked to progressive outer retinal necrosis. The infection typically responds well to intravenous acyclovir therapy.
Fungal infection may be encountered after organ transplantation. In one study, fungal retinitis and endophthalmitis were the most frequent posterior segment infections after BMT, and candida endophthalmitis and aspergillus retinitis were the leading fungal infections.2 The majority of fungal infections were observed within the first two months after BMT. In solid organ transplantation, aspergillus retinitis appears to be more common than candida endophthalmitis.8
 |
Because the primary site of aspergillus infection is subretinal or sub-retinal pigment epithelium, vitreous biopsy may not yield positive results in aspergillosis. In addition to candida and aspergillus, disseminated fusarium infection with secondary fungal endophthalmitis has been reported after organ transplantation.
Toxoplasmic retinochoroiditis may be observed. Although the infection is often a reactivation of existing ocular toxoplasmosis, it may also represent a primary acquired ocular disease due to chronic immunosuppression. The study of cardiac transplant recipients has showed that the infection usually occurs within three to six months and can be bilateral.9 With appropriate antibiotic therapy, the toxoplasmic lesions can be well-controlled. Rarely, toxoplasmic infection in organ transplant recipients may be associated with cerebral dissemination, resulting in significant morbidity and mortality.
Microvascular Retinopathy
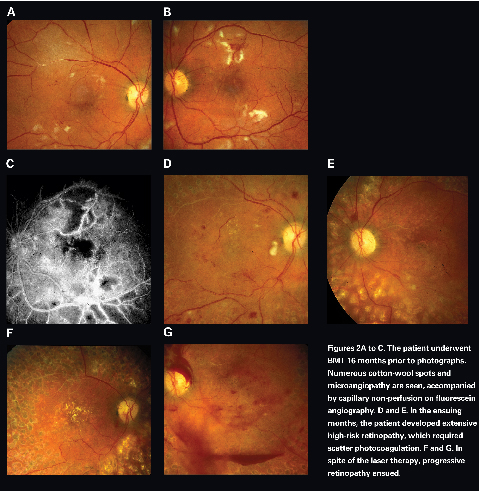
Bone marrow transplantation retinopathy was first reported in 1983.10,11 It typically occurs within six months, but it can occur as late as 62 months after BMT.12-14 Risk factors such as diabetes and hypertension may facilitate the development of BMT retinopathy by heightening the ischemic microvasculopathy. There is no known age, gender or race predilection for development of BMT retinopathye12 Patients present with decreased visual acuity and/or visual field deficit. Posterior segment findings are typically bilateral and symmetric. Clinical manifestations include multiple cotton wool spots, telangiectasia, microaneurysms, macular edema, hard exudates and retinal hemorrhages (See Figure 2). Fluorescein angiography demonstrates capillary nonperfusion and dropout, intraretinal microvascular abnormalities, microaneurysms and macular edema.
Although the precise etiology of BMT retinopathy has not been elucidated, it appears to be affected by several factors: cyclosporine toxicity, total body irradiation (TBI), and chemotherapeutic agents.12,15 Cyclosporine is a powerful immunomodulatory agent that suppresses graft-versus-host immune response. It may lead to endothelial cell injury and neurologic side effects, and as a result, it has been suggested as the cause of BMT retinopathy. However, BMT retinopathy can develop in the absence of cyclosporine use, and cyclosporine has not been shown to cause BMT retinopathy in autologous or syngeneic bone marrow recipients.14 Cyclosporine does not, therefore, appear to be the sole cause of BMT retinopathy.
Total body irradiation has also been implicated as the cause of BMT retinopathy. Radiation injures the retinal microvasculature and leads to ischemic vasculopathy. Variables such as the total dose of radiation and the time interval between radiation and bone marrow ablation appear to be important. However, BMT retinopathy can occur in patients who did not receive TBI, and BMT retinopathy is not observed in solid organ transplant recipients who received similar doses of radiation. Thus, TBI is not the sole cause, but it is another contributing factor in development of BMT retinopathy.
Chemotherapeutic agents have been suggested as a potential contributing factor in BMT retinopathy. Medications such as cisplatin, carmustine, and cyclophosphamide can cause ocular side effects including papilledema, optic neuritis, visual field deficit and cortical blindness.16 It has been suggested that these chemotherapeutic drugs may predispose patients to radiation-induced retinal damages and enhance the deleterious effect of radiation.
In general, patients with BMT retinopathy have a good prognosis. The retinopathy usually resolves within two to four months after stopping or lowering the dosage of cyclosporine.14 In one report, 69 percent of patients experienced complete resolution of the retinal findings, and 46 percent of patients fully recovered their baseline visual acuity.e14 Because of the favorable prognosis and relatively non-progressive nature of BMT retinopathy, aggressive intervention is usually not necessary.
Hematologic Complications
During the early post-transplant period, hematologic abnormalities such as thrombocytopenia, anemia and hyperviscosity may be encountered due to chemotherapy and bone marrow aplasia. These hematologic changes lead to vitreous hemorrhage, intraretinal hemorrhages and other hemorrhagic complications.1 It appears that thrombocytopenia may be the most important factor in the development of posterior segment hemorrhages in these patients. According to one study, vitreous or intraretinal hemorrhagic complications were found in 3.5 percent of patients with BMT, and the majority of hemorrhagic complications were observed within six months.2
Other Complications
Central serous chorioretinopathy (CSCR) may be observed after organ transplantation (See Figure 3). It is more frequently seen after solid organ transplantation than in the setting of BMT. The pathogenesis of CSCR after organ transplantation is likely related to high doses of corticosteroids, emotional stress, hypertension, and cyclosporine. The visual prognosis of CSCR in these patients is generally good, and treatment is indicated only in refractory cases. A study in cardiac and renal transplant recipients revealed that post-transplant CSCR responded well to photocoagulation treatment.17
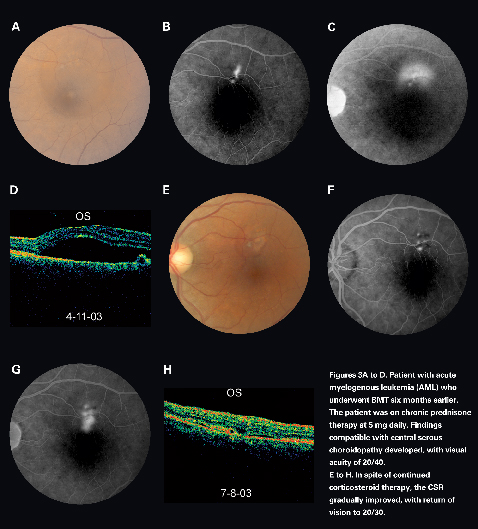 |
Bilateral optic disc edema has been reported after organ transplantation.18,19 It has been attributed to cyclosporine-related neurotoxic effect and/or increased intracranical pressure. One study reported that 2.8 percent of patients developed bilateral optic disc edema after BMT.2
Cyclosporine-induced retinal toxic blindness has been described after BMT.20 In the study, microvascular retinopathy, cortical blindness, and other ocular pathologies were excluded. Electrophysiological studies revealed retinal injury, and the visual acuity improved after the cessation of cyclosporine use.
In summary, posterior segment complications of organ transplantation include infection, microvascular retinopathy, and hemorrhagic findings.21 Other clinical features include CSCR, bilateral optic disc edema, and cyclosporine-related retinal toxicity. These complications may result from the effects of infection, high-dose chemotherapy, cyclosporine, radiation and recurrent malignancies.
Being familiar with these clinical entities is essential in curtailing the potentially sight-threatening complications in organ transplant recipients.
Dr. Moon is vitreoretinal fellow at the Eye Institute, Medical College of Wisconsin, Milwaukee. Dr. Mieler is a professor and chairman of Department of Ophthalmology and Visual Science at the University of Chicago, 5841 So Maryland, MC-2114, Chicago, IL 60637. Contact him at (773) 702-3838; fax: (773) 834-3578; or e-mail: wmieler@uchicago.edu.
The authors received no financial support and have no propriety interest in the topics mentioned.
1. Bray LC, Carey PJ, Proctor SJ, et al. Ocular complications of bone marrow transplantation. Br J Ophthalmol 1991;75:611-614.
2. Coskuncan NM, Jabs DA, Dunn JP, et al. The eye in bone marrow transplantation. VI. Retinal complication. Arch Ophthalmol 1994;112:372-379.
3. Ng P, McCluskey P, McCaughan G, et al. Ocular complications of heart, lung, and liver transplantation. Br J Ophthalmol 1998;82:423-428.
4. Aw MM, Murugasu B, Tan AW, et al. Quantitation of peripheral blood cytomegalovirus DNA for monitoring recurrent cytomegalovirus retinitis in pediatric solid organ transplant recipients. Pediatr Transplant 2000;4:100-106.
5. Larsson K, Lonnqvist B, Ringden O, et al. CMV retinitis after allogeneic bone marrow transplantation: a report of five cases. Transplant Infectious Disease 2002;4:75-79.
6. Hambach L, Stadler M, Dammann E, et al. Increased risk of complicated CMV infection with the use of mycophenolate mofetil in allogeneic stem cell transplantation. Bone Marrow Transplant 2002;29:903-906.
7. Squifflet JP, Legendre C. The economic value of valacyclovir prophylaxis in transplantation. J Infect Dis 2002;186:S116-S122.
8. Rao NA, Hidayat A. A comparative clinicopathologic study of endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared to aspergillosis. Trans Am Ophthalmol Soc 2000;98:183-93.
9. Conrath J, Mouly-Bandini A, Collart F, et al. Toxoplasma gondii retinochoroiditis after cardiac transplantation. Graefes Arch Clin Exp Ophthalmol 2003;241:334-338.
10. Hirst LW, Jabs DA, Tutschka PJ, et al. The eye in bone marrow transplantation. I. Clinical study. Arch Ophthalmol 1983;101:580-584.
11. Gratwhol A, Gloor B, Hahn H, Speck B. Retinal cotton wool patches in bone marrow transplant recipients. N Engl J Med 1983;308:1101.
12. Gomez-Ulla F, Rodriguez-Cid MJ, Gomez-Torreiro M, et al. Bone marrow transplantation retinopathy. Int Ophthalmol 2001;24:33-35.
13. Bylsma GW, Hall AJ, Szer J, et al. Atypical retinal microvasculopathy after bone marrow transplantation. Clin Exp Ophthalmol 2001;29:225-229.
14. Bernauer W, Gratwohl A, Keller A, et al. Microvasculopathy in the ocular fundus after bone marrow transplantation. Ann Intern Med 1991;115:925-930.
15. Lopez PF, Sternberg P, Dabbs CK, et al. Bone marrow transplant retinopathy. Am J Ophthalmol 1991;112:635-646.
16. Khawly JA, Rubin P, Petros W, et al. Retinopathy and optic neuropathy in bone marrow transplantation for breast cancer. Ophthalmol 1996;103:87-95.
17. Friberg TR, Eller AW. Serous retinal detachment resembling central serous chorioretinopathy following organ transplantation. Graefes Arch Clin Exp Ophthalmol 1990;228:305-309.
18. Avery R, Jabs DA, Wingard JR, et al. Optic disc edema after bone marrow transplantation: possible role of cyclosporine toxicity. Ophthalmol 1991;98:1294-1301.
19. Walter SH, Bertz H, Gerling J. Bilateral optic neuropathy after bone marrow transplantation and cyclosporin A therapy. Graefes Arch Clin Exp Ophthalmol 2000;238:472-476.
20. Lopez-Jimenez J, Sanchez A, Fernandez CS, et al. Cyclosporine-induced retinal toxic blindness. Bone Marrow Transplant 1997;20:243-245.
21. Moon SJ, Mieler WF: Retinal complications of bone marrow and solid organ transplantation. Curr Opin Ophthalmol 2003;14:433-442.



