Ancillary imaging with fluorescein angiography, ocular ultrasonography and optical coherence tomography were obtained. On FA, the left eye showed temporal and inferior telangiectasia and peripheral non-perfusion without traction (Figure 1C). Ocular ultrasonography disclosed shallow retinal detachment with subretinal dense debris and no calcific mass. There was a retinal macrocyst superiorly. OCT revealed the fovea overlying subretinal dense, irregular debris with loss of the outer retinal layers and trace cystoid macular edema (Figure 1D).
The differential diagnosis in a 2-year-old patient with exudative retinopathy includes a wide range of pathologies, including Coats’ disease, retinal hemangioblastoma, vasoproliferative tumor, familial exudative vitreoretinopathy, facioscapulohumeral muscular dystrophy, retinopathy of prematurity sequelae, retinitis pigmentosa and some infections. Rarely does retinoblastoma display exudative retinopathy. Less-common causes include diabetes mellitus and hypertension. While the patient’s history of prematurity with NICU admission might prompt concern for late sequelae of retinopathy of prematurity, the dilated fundus examination was less consistent as there was an absence of tractional elements of extraretinal fibrovascular tissue or vitreoretinal scarring. Instead, the presence of extensive retinal telangiectasia associated with retinal exudation, with no sign of retinal traction was most suggestive of Coats’ disease. Given the absence of prior medical or family history, the clinical diagnosis of Coats’ disease was established and the patient underwent laser photocoagulation and cryotherapy to the areas of telangiectasia.
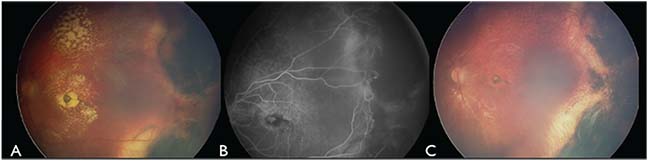 |
| Figure 2. (A) Fundus photography of the left eye four months after first cryotherapy demonstrates partial resolution of the regions of retinal exudation, chorioretinal scarring in areas of cryotherapy and residual telangiectasia along the posterior margin. (B) Fluorescein angiography during the same visit highlights the residual telangiectasia adjacent to the chorioretinal scarring temporally. (C) Fundus photography at one year after treatment shows subfoveal gliotic scar, temporal chorioretinal scarring and resolution of the telangiectasia. |
On follow-up examination four months later, there was 60-percent resolution of the exudation, with temporal chorioretinal scarring, mild persistent subretinal fluid and residual telangiectasia along the posterior margin of the previous treatment temporally (Figure 2A). FA confirmed the presence of residual telangiectasia (Figure 2B). A second round of therapy was then performed over these remaining telangiectasia.
On follow-up six months after the the second treatment, funduscopy revealed minimal macular exudation with a residual foveal gliotic scar and peripheral treatment scarring. FA confirmed inactive telangiectasia and a macular blocking defect from gliosis. OCT demonstrated the fovea draped over old exudation and an otherwise flat retina. No further intervention was performed, and the most recent follow-up 12 months after the second round of treatment showed complete resolution of the telangiectasia and macular exudation, with persistent foveal gliosis (Figure 2C).
Discussion
Coats’ disease was first described by George Coats in 1908 as unilateral retinal vascular abnormalities with retinal exudation, typically found in young males.1 The definition has since been refined to findings of idiopathic retinal telangiectasia with intraretinal and/or subretinal exudation without evidence of vitreoretinal traction.2,3 Coats’ is rare and sporadic, and hasn’t been linked to any systemic diseases. A one-year, prospective survey in the United Kingdom estimated a population incidence of 0.09 per 100,000.4 This condition typically presents unilaterally in younger males: In a study of 150 patients with Coats’ disease, 76 percent were males and 95 percent presented with unilateral findings. The median age at presentation was 5 years (age range: 1 month to 63 years).3
The most common initial presenting symptoms of Coats’ disease included decreased visual acuity, strabismus and leukocoria.3 In the natural course of the disease, retinal telangiectasia and associated vascular abnormalities, including microaneurysms, arteriovenous communications and capillary dropout lead to extensive vascular leakage with subsequent subretinal fluid and exudation. Interestingly, while most telangiectasia are found temporally and anterior to the macula, exudation is often found more diffusely involving the periphery and post-equatorial retina.3,5 Exudation in Coats’ disease is often remote from the areas of telangiectasia and has a notable preference for the macula, forming a macular star.2,3,5,6 As subretinal fluid progresses, retinal detachment can occur and may progress to total detachment.
Coats’ disease is primarily diagnosed clinically, though several diagnostic procedures can assist in unclear cases. Fluorescein angiography highlights telangiectasia with early hyperfluorescence, hypofluorescence of exudation, capillary dropout peripheral to telangiectasia, and mild hyperfluorescence of subretinal fluid and overlying retinal capillaries. Ultrasonography can be particularly helpful in advanced cases with total exudative retinal detachment. Subretinal fluid will typically be clear or with minor, noncalcific echogenicity from subretinal cholesterolosis. These findings are particularly helpful in differentiating Coats’ from the solid mass with dense echoes of calcification seen in retinoblastoma. In unusual cases, cytological assessment of the subretinal fluid can show lipid-laden macrophages and cholesterol crystals.3,7
The progression of Coats’ disease has been classified into five stages. Stage 1 involves only retinal telangiectasias. Stage 2 includes additional exudation, with further subclassification specifying extrafoveal exudation as 2A and intrafoveal exudation as 2B. In stage 3, additional exudative retinal detachment is noted with subclassification detailing partial and total retinal detachment as 3A and 3B, respectively. Stage 3A is further subclassified into extrafoveal and foveal partial detachment as 3A1 and 3A2, respectively. Stage 4 describes eyes with total retinal detachment and secondary glaucoma. In stage 5, end-stage disease has occurred with a blind, nonpainful or painful eye and total retinal detachment. In a review of 124 eyes, the following incidences across stages were noted: stage 1 (1-percent incidence); stage 2 (14 percent); stage 3 (68 percent); stage 4 (15 percent); and stage 5 (2 percent).2 Recently, the subfoveal gliotic nodule has been found to be predictive of macular fibrotic scarring and poorer visual outcomes.8
The management of Coats’ disease is stage-dependent. Stage 1 or early stage 2 disease with mild telangiectasias and only small amounts of exudation are either observed or treated with laser photocoagulation. Observation is especially considered in older patients where the disease is usually less aggressive. Also, spontaneous regression of Coats’ disease has been described.9,10 However, periodic follow-up should be maintained. In a retrospective study of 39 patients across two decades, there was a trend toward better final visual acuity in the second decade of life when eyes were treated more often and with a higher number of procedures, suggesting that a low threshold for treatment is best.11 Appropriate treatment has been shown to anatomically stabilize or improve the eye in 76 percent of eyes.2
For stage 2 Coats’ disease, treatment modalities include laser photocoagulation or cryotherapy directly to the area of telangiectasia.2,12,13 Multiple treatments may be required, and areas of remote exudation often resolve with the telangiectasia. Both modalities may also be used in some cases of partial retinal detachment. In eyes with total retinal detachment, visual prognosis is considered poor11,14 and the decision against intervention must be weighed against the risk of developing painful neovascular glaucoma. In two retrospective case reviews of advanced Coats’ disease that were untreated, four of six and 18 of 25 patients progressed to neovascular glaucoma.14,15 Considering this risk, vitrectomy with subretinal fluid drainage, intravitreal tamponade with silicone oil or gas, and concomitant cryotherapy or photocoagulation to the telangiectasia has been performed with variable success, with the goal of preventing development of painful neovascular glaucoma.2,12,13,16 Others have used treatments such as intravitreal triamcinolone or repetitive laser photocoagulation directly to telangiectasia on the detached retina.17,18 Enucleation may be required in advanced disease that has progressed to a blind painful eye with or without secondary glaucoma. For stage 5 disease, in the absence of pain, observation is advised.
The use of anti-VEGF has been proposed as an adjunctive therapy for Coats’ disease.11,19-22 VEGF has been found to be elevated in the disease23 and a number of case series have described its use in Coats’ with favorable results.11,19,21,22,24 In one small series of 10 patients treated with intravitreal bevacizumab compared to 10 matched controls, all of the bevacizumab patients achieved full resolution of the disease, compared to eight out of 10 in the control group.20 The effects of anti-VEGF therapy in Coats’ disease remains under study, and there are several limitations associated with it: One small study noted the formation of tractional fibrosis (rarely seen in Coats’ disease) in four out of eight patients treated with intravitreal bevacizumab.24 The use of anti-VEGF in the pediatric population may also be of concern, given the potential for systemic effects in developing children with relatively small volumes of distribution. There is also the risk of mistakenly injecting a patient with undiagnosed retinoblastoma, leading to potentially catastrophic consequences.25
The outcome of Coats’ disease is particularly stage-dependent. In a study of 124 treated eyes, post-treatment poor visual acuity of 20/200 or worse was found in zero percent of stage 1, 53 percent of stage 2, 74 percent of stage 3 and 100 percent of stages 4 and 5. Enucleation was required in 0 percent of stages 1 and 2, 7 percent of stage 3, 78 percent of stage 4 and zero percent of stage 5. Poor visual acuity was associated with post-equatorial or diffuse location of the telangiectasia, retinal macrocyst and failed resolution of subretinal fluid after treatment.2 It’s important to note that Coats’ disease can recur. In a retrospective analysis performed over 25 years, there was recurrence of telangiectasia and exudation in six out of 86 eyes (7 percent) that had initially been treated with good control of the disease.2
In conclusion, Coats’ disease is a rare, nonhereditary condition defined by idiopathic retinal telangiectasia with retinal exudation and without evidence of retinal or vitreal traction. This condition typically presents unilaterally in young males, and a high index of suspicion should be maintained in such patients presenting with leukocoria and/or decreased vision. REVIEW
1. Coats G. Forms of retinal diseases with massive exudation. Royal London Ophthalmology Hospital Reports;1908:17:440-525.
2. Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: The 2000 Proctor Lecture. Am J Ophthalmol 2001;131:5:572-583.
3. Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: The 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol 2001;131:5:561-571.
4. Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: Epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24:12:1797-1801.
5. Egerer I, Tasman W, Tomer TT. Coats disease. Arch Ophthalmol 1974;92:2:109-112.
6. Ridley ME, Shields JA, Brown GC, Tasman W. Coats’ disease. Evaluation of management. Ophthalmology 1982;89:12:1381-1387.
7. Eagle R. Eye Pathology: An Atlas and Text. Third ed. Philadelphia: Wolters Kluwer; 2017.
8. Daruich AL, Moulin AP, Tran HV, Matet A, Munier FL. Subfoveal nodule in Coats’ disease: Toward an Updated Classification Predicting Visual Prognosis. Retina 2017;37:8:1591-1598.
9. Deutsch TA, Rabb MF, Jampol LM. Spontaneous regression of retinal lesions in Coats’ disease. Can J Ophthalmol 1982;17:4:169-172.
10. Wolfe JD, Hubbard GB. Spontaneous regression of subretinal exudate in Coats’ disease. Arch Ophthalmol 2006;124:8:1208-1209.
11. Ong SS, Buckley EG, McCuen BW, et al. Comparison of Visual Outcomes in Coats’ Disease: A 20-Year Experience. Ophthalmology 2017;124:9:1368-1376.
12. London NJS, Shields CL, Haller JA. Chapter 56 - Coats Disease. In: Schachat A, ed. Retina (Fifth Edition). London: W.B. Saunders, 2013:1058-1070.
13. Mulvihill A, Morris B. A population-based study of Coats disease in the United Kingdom II: Investigation, treatment, and outcomes. Eye (Lond) 2010;24:12:1802-1807.
14. Haik BG. Advanced Coats’ disease. Trans Am Ophthalmol Soc 1991;89:371-476.
15. Silodor SW, Augsburger JJ, Shields JA, Tasman W. Natural history and management of advanced Coats’ disease. Ophthalmic Surg 1988;19:2:89-93.
16. Yoshizumi MO, Kreiger AE, Lewis H, Foxman B, Hakakha BA. Vitrectomy techniques in late-stage Coats’-like exudative retinal detachment. Doc Ophthalmol 1995;90:4:387-394.
17. Levinson JD, Hubbard GB. 577-nm yellow laser photocoagulation for Coats disease. Retina 2016;36:7:1388-1394.
18. Ghazi NG, Al Shamsi H, Larsson J, Abboud E. Intravitreal triamcinolone in Coats’ disease. Ophthalmology 2012;119:3:648-649.
19. Lin CJ, Hwang JF, Chen YT, Chen SN. The effect of intravitreal bevacizumab in the treatment of Coats disease in children. Retina 2010;30:4:617-622.
20. Ray R, Barañano DE, Hubbard GB. Treatment of Coats’ disease with intravitreal bevacizumab. Br J Ophthalmol 2013;97:3:272-277.
21. Villegas VM, Gold AS, Berrocal AM, Murray TG. Advanced Coats’ disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin Ophthalmol 2014;8:973-976.
22. Zheng XX, Jiang YR. The effect of intravitreal bevacizumab injection as the initial treatment for Coats’ disease. Graefes Arch Clin Exp Ophthalmol 2014;252:1:35-42.
23. He YG, Wang H, Zhao B, Lee J, Bahl D, McCluskey J. Elevated vascular endothelial growth factor level in Coats’ disease and possible therapeutic role of bevacizumab. Graefes Arch Clin Exp Ophthalmol 2010;248:10:1519-1521.
24. Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96:3:356-359.
25. Avery RL. Extrapolating anti-vascular endothelial growth factor therapy into pediatric ophthalmology: Promise and concern. J AAPOS. 2009;13:4:329-331.



