Patients who need anti-VEGF injections shoulder a significant burden that has retinal specialists increasingly concerned, especially when some patients lose hope and their commitment to treatment wanes. So says David Boyer, MD, a senior partner at the Retina-Vitreous Associates Medical Group, based in Los Angeles and surrounding areas. “This is like getting chemo,” he adds. “These patients have to make visits every six or seven weeks. Some of them have other medical conditions that require doctor visits. After three years or so, they get tired and some start taking a chance to see if they can get by without a visit. If they start to bleed, we can’t get them back to where they were.”
With FDA approval in October 2019 of brolucizumab (Beovu, Novartis)—ostensibly the most durable anti-VEGF drug of five that have been used during the past 15 years and the first to be approved since 2011—ophthalmologists and patients look to a future when the burden of continual anti-VEGF injections lightens. Many therapeutic improvements in the treatment of retinal diseases are expected during the next five years, beginning with the roll-out of Beovu, expected to keep more than half of patients stable and treatment-free for up to three months. You can also expect longer-acting agents, combination strategies, topical agents, a sustained-release implant and genetic therapies to lessen the treatment burden.1
In the war against blinding diseases, however, progress continues slowly, one clinical trial at a time. To improve the treatment approach, retinal experts here discuss the use of Beovu and existing anti-VEGF agents, and how to keep patients motivated while maintaining optimal vision.
Patient Struggles
Dr. Boyer says patients’ struggles against wet age-related macular degeneration, diabetic macular edema and central retinal vein occlusion are getting more difficult. “We need to pay more attention to the burden of treatment on patients and their families,” he notes. “As we know, diabetic patients, most of them working-age people, face the biggest challenges. If they need to come in for injections once a month, that can amount to their total vacation days at work.”
A European study reviewed the burden on 131 patients with diabetic macular edema and central vein occlusion who who were undergoing anti-VEGF therapy in 2015 and 2016.2 The findings revealed that each injection appointment (including travel time) took an average of 4.5 hours. For each six-month period of treatment, more than 13 hours were needed for RVO visits and 20 hours were dedicated to DME appointments. A total of 53 percent of working patients needed to take off at least one day per appointment, and 71 percent of all patients required assistance with travel and personal needs.
 |
An Even Worse Burden
“Beyond the burden that affects DME, CVO and wet AMD patients, undertreatment quickly turns into an even worse burden,” Dr. Boyer continues. “Evidence points to a continuing problem with patients receiving fewer anti-VEGF injection treatments than recommended in the long-term—and a commensurate decrease in visual acuity in many patients. We can attribute this to a number of factors, including poor compliance and insufficient monitoring.”3,4,5
Besides diligently following patients and encouraging their compliance with visits, Dr. Boyer and his colleagues believe more durable anti-VEGF agents and alternative therapies in the future—such as the implantable port delivery system being developed by Genentech and gene therapy—will open the door to better outcomes and tolerable burdens for patients. These innovations should also help reduce the often overwhelming patient flow in retinal offices, he adds. For now, Beovu, the latest addition to the anti-wet-AMD arsenal, should help keep many patients away from the needle for three months at a time, following a three-month loading phase. Specialists say they’re planning to begin using it as soon as they receive initial deliveries and finalize reimbursement issues. A permanent J-code for Beovu has already been assigned and should go into effect this month.
Beovu was approved following the Phase III HAWK and HARRIER studies,6 double-masked, multicenter, head-to-head trials involving more than 1,800 patients with wet AMD across nearly 400 centers around the world. The trials compared the efficacy and safety of Beovu 6 mg (both studies) and 3 mg (HAWK only) versus aflibercept (Eylea) 2 mg in wet AMD patients. Beovu met its primary endpoint of noninferiority versus Eylea in mean change in best-corrected visual acuity at year one, even though more than half of patients were treated every 12 weeks, compared to every eight weeks with Eylea during the maintenance phase. Beovu, with its single-strand antibody fragment composition, delivers a high concentration of drug to the target area and achieves greater reduction in central subfield thickness and subretinal fluid than Eylea.
“We don’t know yet how much of a change Beovu will make in our management of patients,” says Dr. Boyer. “If the drug can dry out patients faster, as the evidence suggests it does, that will be positive. But our use of it in practice might not reflect the exact approach that was used in the studies. We may see an extension of a few weeks over what we see now. Depending on clinical response, vision and OCT readings, we can already achieve up to three-month intervals for ranibizumab (Lucentis) and Eylea. The real game changers in controlling AMD have yet to come.”
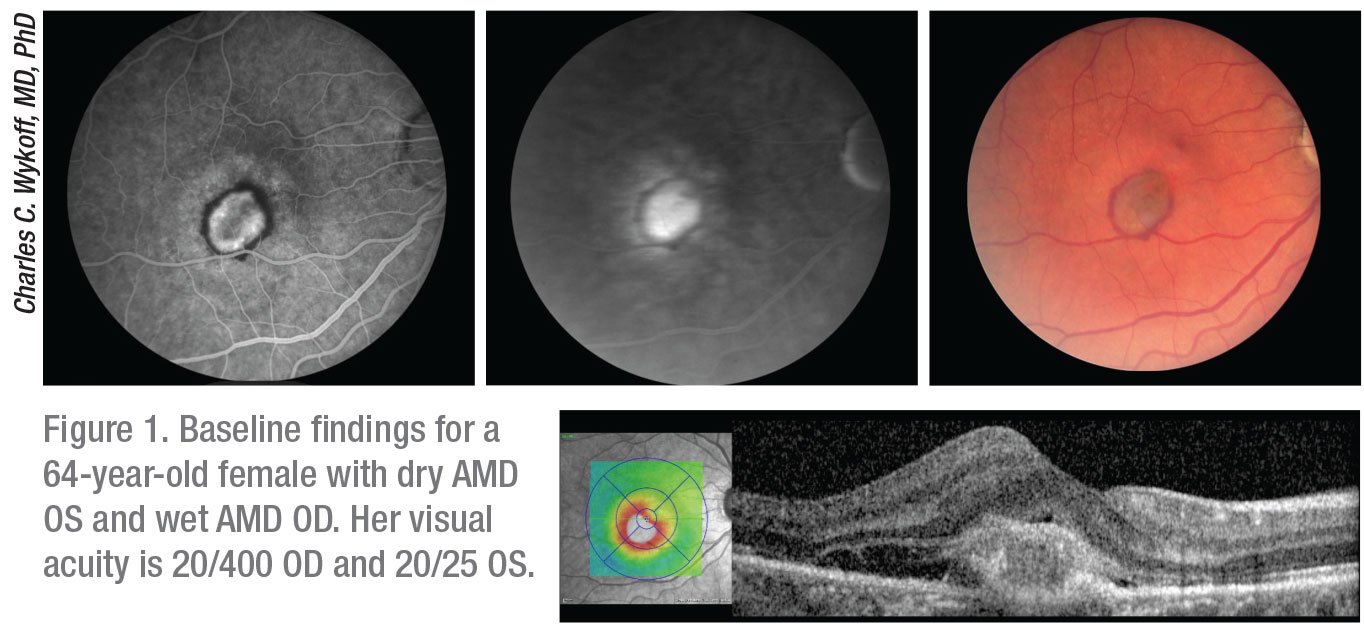
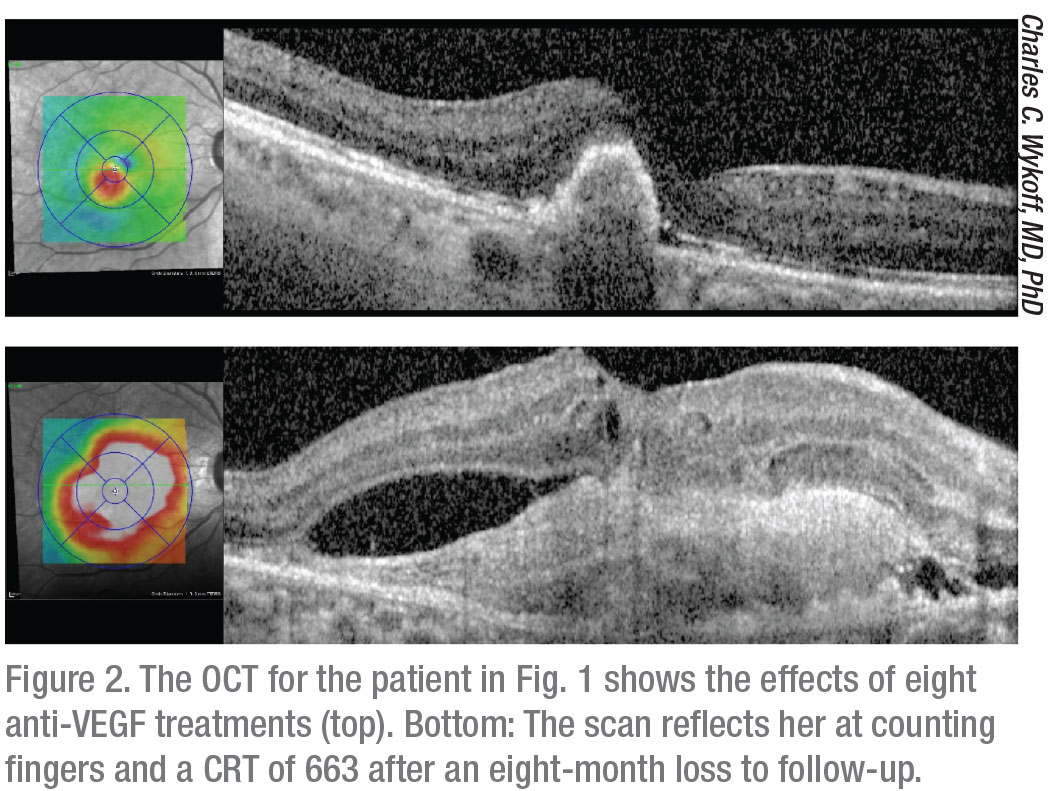 |
Individualized Care
With an aim toward easing treatment burden while ensuring effective care, Jennifer Lim, MD, director of the Retina Service at the University of Illinois-Chicago College of Medicine, believes Beovu will increase her treatment options based on meeting individual needs. “I think identifying a personalized treatment interval for a patient is reasonable,” she says. “That is, don’t just use the interval used for a patient who might have fit into a particular cohort of a clinical trial.
“In my practice, I see a patient, treat the eye, re-evaluate how the patient is doing a month later and continue to treat monthly until the patient stabilizes,” she continues. “Then, I begin to extend treatment, following the patient specifically for her or his disease. I look at visual acuity, OCT scans and clinical exam findings, then decide—again, for that patient— how I am going to treat. I typically extend first for two weeks. If the patient’s still doing well, I extend the patient further and eventually push for the maximal interval I can achieve for that patient.”
When considering how she might incorporate Beovu in her practice, Dr. Lim says: “This new medication doesn’t have clinical trial data that shows you can extend out in a vast majority of patients. In the HAWK and HARRIER trials, investigators were able to extend the treatment interval to about 12 weeks for 56 percent in one study and 51 percent in the other.”
Although this is a significant benefit, she recommends putting the findings in the context of other medications used on an off-label basis. “Keep in mind that other trials have also shown quarterly dosing to be effective. I’ve even managed to extend patients longer than three months. For example, consider the ranibizumab (Lucentis) data from the HARBOR study, which involved p.r.n dosing.7 Only 7 percent required monthly dosing, and the average interval was almost 10 weeks when extending treatment.”
Dr. Lim notes that once she’s established a treatment regimen for patients, she hardly brings any of them back monthly to her clinic, decreasing the burden of injections as much as possible. “It’s most likely the same for other practices,” she adds. “And a significant portion of this small subgroup consists of polypoidal variant patients, who are a little bit harder to treat, or those with pigmentary epithelial detachments, who can be very difficult to treat.”
Dr. Lim doesn’t rely solely on trial data to inform her use of any agents. “The bottom line is that, in this day and age, we should be trying to personalize treatment and not just say ‘monthly is best.’ If you watch your patient closely enough, you won’t lose her because of undertreatment—provided she is reliable and returns for follow-up visits.”
Which type of patient would she say is ideal for Beovu? “A patient you’ve been extending and treating, but whom you can only extend so far because of recurrent fluid,” says Dr. Lim. “In both the HAWK and HARRIER trials for Beovu, investigators found drier retinas (on OCT) sooner than with Elyea. So these types of patients would present an ideal opportunity. If you follow up at two months and find the patient is dry, then maybe you can try to extend the patient to see him back in another two months. Then push the envelope from there.”
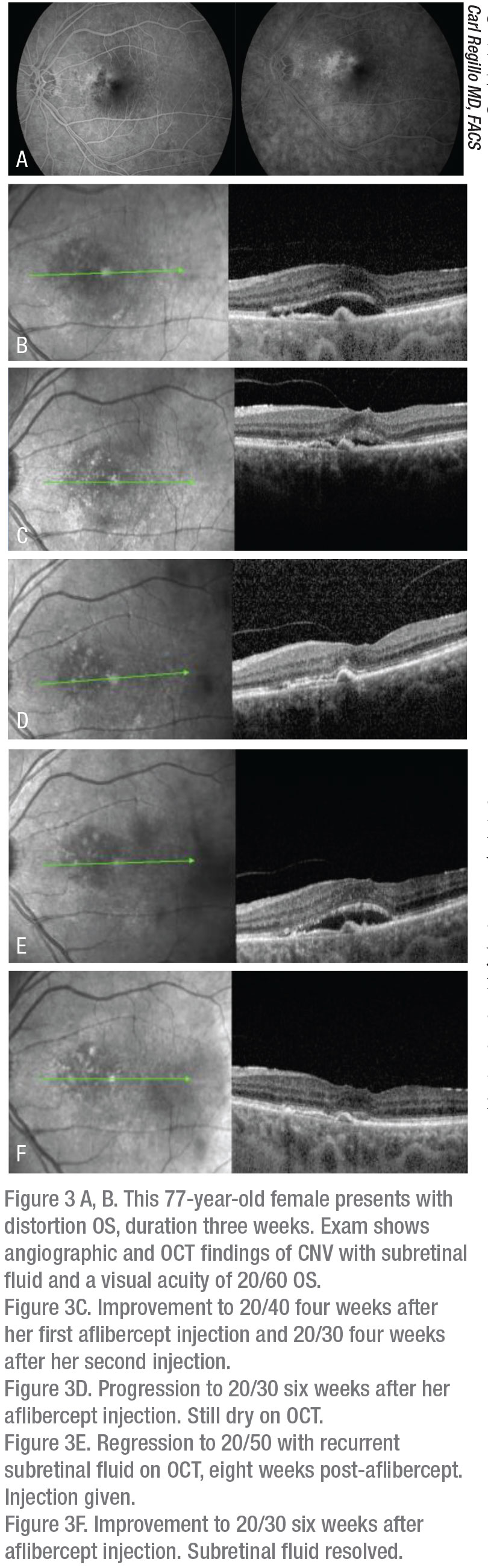 |
“Can We Do Better?”
Even with the approval of Beovu, leading retinal specialist Charles C. Wykoff, MD, PhD, says significant progress is still needed. “The anti-VEGF agents that we have are excellent,” says Dr. Wykoff, the associate professor of clinical ophthalmology, Institute for Academic Medicine, and associate clinical member of the Research Institute, Houston Methodist and Weill Cornell Medicine. “But the need for repeated, long-term injections remains a major challenge for patients, payors and physicians. Many patients with wet AMD aren’t receiving the doses they need to achieve optimal anatomic and visual outcomes. So we clearly have a disconnect between how effective these medications can be and how they’re being utilized and, therefore, we have a disconnect in outcomes. We know more durable agents, such as the ones in the future, will be very valuable. We can expect increased durability immediately from Beovu. But the question is: Can we do better than that? Can we go from 35 to 45 percent of the patients at two years being at quarterly dosing to 70, 80 or 90 percent?”
Provided insurance pays for Beovu, 50.3 percent of U.S. retinal physicians say they’ll use the agent for incomplete responders to other anti-VEGF agents, according to the 2019 American Society of Retina Specialists Preferences and Trends (PAT) Survey.8 A total of 41.1 percent of PAT respondents will take a gradual approach to Beovu, using it on some patients and expanding its use if they see beneficial effects.
“Vision, of course, is the most important end point for patients and physicians,” says Dr. Wykoff. “Most of our day-to-day decision-making on management and treatment paradigms is based on OCT and anatomy. So you may have patients with hemorrhages on OCT, or subretinal or intraretinal fluid. And then other findings come into play. Do they have geographic atrophy or a lot of geographic atrophy in only one eye? You may end up dosing them less frequently in the poorer-seeing eye. In the better-seeing eye, you may find residual fluid, and those are the eyes where you’re going to be more aggressive to maintain as much vision as possible.”
Immediate Impact
Carl Regillo, MD, chief of the retina service at Wills Eye Hospital in Philadelphia, expects Beovu to have an immediate impact on treatment burden. “The medication allows for the potential to extend the treatment interval for our patients with wet AMD, regardless of their current treatment interval using one of our other agents, such as ranibizumab (Lucentis), bevacizumab (Avastin) or Eylea,” he says. “We have a large population of wet AMD patients, most of whom are doing well with our current drugs. For any given patient, we usually can’t extend them beyond a certain point. The time period varies, and could be four, eight or 12 weeks for the patient. This drug gives us the potential to switch those patients to a drug that appears to be a little more durable, extending that treatment. The additional extension could add another two or three weeks or so to a patient’s treatment interval. That could become one or two less treatments a year, saving on co-payments, travel and inconvenience.”
When considering the other anti-VEGF agents, Dr. Regillo says studies, and his experience, indicate that Eylea is slightly more durable than Lucentis. “Elyea dries a little better under certain circumstances,” he observes. “But in wet AMD, those small differences don’t really bear out much in head-to-head testing. So across the board, there’s very little difference between these two drugs for wet AMD patients. Across the board for Beovu, we’re talking about more durability. Not a lot more, but enough to see it across the board. That’s why I think anyone is a potential candidate for use of this drug. You could say that Eylea has had some slight advantages that studies didn’t really show convincingly. But here, we have studies that show definitively better drying in wet AMD, which wasn’t shown to be different among the drugs previously.”
Dr. Regillo says he sees Beovu easing the burden for up to half of patients in his practice. “This therapy will benefit wet AMD patients who are now achieving four to six to eight week intervals. The medium interval is about eight weeks for them. We should be able to extend all of them a bit.” He notes that the benefit of Beovu may not mean as much to patients who are already up to 12-to-14-week intervals. “They may not be as motivated to switch their current regimens for such a small gain. But we’ll have to see how it plays out.”
Decreasing the frequency of office visits could significantly appeal to lower-interval patients, he notes. “It helps when they have more latitude. The longer we can extend, the more flexibility they have while maintaining vision gains over time. Barriers—such as comorbidities, out-of-pocket expense, getting to the office—are all factors that affect compliance.”
Dr. Boyer says his patients will take advantage of any “time off” from regular visits, no matter how limited the gain. “I’m thinking of the patients who do treat-and-extend, as opposed to p.r.n. and monthly,” he says. “If you can get an extra week, you can save a few injections over a two-year period, and they appreciate that.”
Maximizing All Therapies
Dr. Wykoff says retinal specialists are also exploring the maximum potential durability of Eylea, Lucentis and Avastin. “Between 22 and 42 percent of eyes can ultimately be treated quarterly with current anti-VEGF drugs,” he notes. “That doesn’t include Beovu, with its impressive data. A meaningful proportion of our patients can already be extended and treated quarterly once their eyes are dry and stable. So while we look forward to more durable agents, we need to realize that current agents are quite durable for many patients.”
Like Dr. Lim, Dr. Wykoff also recommends an individualized approach, depending on the eye. “If there’s a small AMD lesion that dries quickly with anti-VEGF therapy, the eye can often can be treated very infrequently and do very well. The larger lesions with more persistent fluid tend to require more aggressive regular treatment, even monthly dosing. We’ve taken individualized approaches for years. Now, more than ever, prospective trials and discussions with patients can help inform our decisions.”
Keep Them Coming Back
Retinal specialists have tried a number of approaches to reduce treatment burden. For example, in 2007, according to the PAT Survey, nearly 80 percent of anti-VEGF patients received only one injection per eye for each visit. By 2019, that percentage decreased to less than 35 percent. Now, more than 60 percent of patients receive injections in both eyes when they visit offices and clinics.8
Regarding compliance, subspecialists say you can achieve more impact than you might suspect. Dr. Regillo’s practice has hired a person to follow up with patients who miss appointments. “Having somebody call patients to find out why they missed appointments helps,” he says. “Not only can we remind them of the importance of all of their visits, we can see if they need assistance. Transportation or a health problem could be issues. They may not be aware of transportation programs and other programs available in the community.”
Dr. Boyer says increasing the efficiency of each visit and patient education are critical. “We try to get patients in and out quickly,” he says. “However, you still need to thoroughly explain what’s going on and answer questions. For example, when the extent of atrophic damage from dry AMD increases, even while we’re treating wet AMD, patients may be disappointed because their anti-VEGF seems like a failure.”
Dr. Lim relies on her patients’ input as one way of keeping them engaged and, as a result, more compliant. “You can use treat-and-extend for patients who don’t want to keep coming back that often,” she says. “But I also have patients who say they want to come back every month because they want to figure out what their sweet spot is. If they want to come back every month to determine whether or not they need an injection, I’ll do it. Patients can help sway you, and there is nothing wrong with taking that approach. Then there are reliable, relatively healthy patients who want to be seen every two months, once we determine they need treatments at that interval. I treat as needed. But I only offer p.r.n treatments to patients who are extremely reliable and relatively healthy and unlikely to miss an appointment because of illness, short of a catastrophic event.”
Dr. Wykoff says his practice relies on “safety nets” if patients miss their visits. “We make phone calls to them. We help them get rides,” he says. “We’re all about trying to make it as easy as possible to get in and get treatment. Many patients travel, and we’ll coordinate to get them their treatments, even if they go abroad.”
He says effective communication is the most important strategy. “We communicate early and often with patients and their families,” he says. “I also try to communicate the promise and hope of ongoing research and future possible opportunities. Many patients ask, ‘So I’ll need these shots forever?’ I say, ‘Forever for now. We’ll do better eventually. This is the best we can offer. Thank goodness we have it, though, because it’s preventing people from going blind.’ ” REVIEW
Dr. Lim serves on the speakers bureau, as a consultant and is involved in grants/research for Genentech. She is also a consultant for Novartis and is involved in grants/research for Regeneron, Grayburg, Stealth, Chengdu Kanghong and Alderya. She is a consultant for Ophthea, Aura and Santen, and she receives honoraria for meetings from Alcon, PSivida and Allergan. Dr. Boyer is a consultant for Allergan, Novartis, Genentech, Regeneron, Adverum, Humera, Roche and Graybug. Dr. Wykoff is involved in research and consulting with Adverum, Allergan, Apellis, Chengdu Kanghong, Clearside Biomedical, Genentech/Roche, Iveric Bio, Kodiak, Novartis and RegenXBio. He’s also a consultant for Alimera Sciences and Bayer. Dr. Regillo is a consultant and is involved in research/grants for Allergan, Chengdu Kanghong, Genentech, Kodiak and Novartis.
1. Al-Khersan H, Hussain RM, Ciulla TA2,, Dugel PU. Innovative therapies for neovascular age-related macular degeneration. Expert Opin Pharmacother 2019;20;15:1879-1891.
2. Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol 2016;10:939-46.
3. Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Oph-thalmol. 2014 Apr;157:4:825-833.
4. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:2:220-226.
5. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013 Nov;120:11:2292-9.
6. Markham A. Brolucizumab: First approval. Drugs. 2019;79:18:1997-2000.
7. Ho AC, Busbee BG, Regillo CD, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014 Nov;121:11:2181-92.
8. Stone TW, Hahn P, eds. ASRS 2019 Preferences and Trends Membership Survey: Chicago, IL. American Society of Retina Specialists; 2019.