Imagine this: You've just performed successful, uncomplicated cataract surgery. Your patient is 20/20 and the surgery looks beautiful; you're ready to be congratulated. Instead, your patient says, "I hate it! These unwanted images are driving me crazy! You've got to do something about this."
Of course, this isn't what you want to hear. But the reality is that dysphotopsia has become the number one problem following uncomplicated, successful cataract surgery. And it doesn't go away easily once a patient becomes focused on it. In my experience, if a patient has perceived this as a major problem for three months or more, it won't improve without intervention.
Unfortunately, many of these patients are incredibly unhappy. Quite a few end up in my office after seeing five or six other doctors, most of whom have told the patients that they're crazy. "Your surgery is perfect," they say. "There's nothing wrong here." This has entirely the wrong effect, making the patient angrier and even more focused on the unwanted images.
How upset are these patients? Some are angry enough to sue. I was recently asked to be an expert witness in a class action lawsuit against a major lens manufacturer in connection with dysphotopsia. (I declined.) I've also heard that there's a website for patients who are miserable because of these symptoms, giving them a forum to share their anger.
Here, I'd like to talk about the different aspects of the problem and review strategies that can help resolve your patient's complaint—and share a few things that you definitely don't want to do.
The Nature of the Problem
In terms of numbers, based on my experience and that of my colleagues, the number of patients who actually require an intraocular lens exchange because of dysphotopsia is only about one in a thousand. However, the number of patients complaining about dysphotopsia is closer to one in ten. It's also worth noting that the percentage of patients complaining depends upon whether you ask them about this problem. A lot of people are unhappy; they just don't say anything to the doctor. (This is not a good thing.)
In the past, most of the problems patients had along these lines were edge-related visual symptoms. These were primarily reflections off the intraocular lens's truncated edge, most often problematic in lenses with a higher refractive index. (See Figure 1).
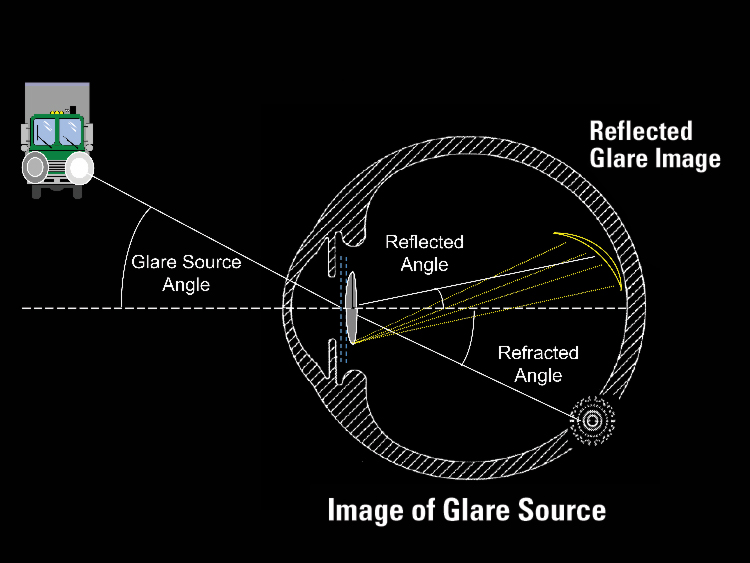 |
| Figure 1. Square-edge IOL design appears to be the primary cause of reflected nighttime glare. However, positive dysphotopsias seldom result in lens replacement. Negative dysphotopsias—perceived dark areas—are more difficult for patients to tolerate. |
Fortunately, advances in lens edge design have minimized this problem. Some surgeons believe that the new edge designs also help to relieve negative dysphotopsia (perceiving a dark area in the periphery of vision), but in my ex-perience it's a separate problem. The fact that dysphotopsia remains an issue supports this conclusion.
So what's behind the current wave of dysphotopsia complaints? The problem really has two distinct elements:
• The first element is what the patient is actually seeing. We can take steps to minimize the unwanted images in many instances, but we can't always eliminate them.
• The second element is how the patient reacts to the symptom. Be-cause the brain is an integral part of the vision process, the patient's reaction can be the most significant factor in resolving (or not resolving) the problem.
What the Patient Sees
The specific visual symptoms reported by these patients may include temporal darkness, shimmering or pulsing light, arc, flare, and/or a central flash (usually an edge reflection caused by a peripheral light).
• Temporal darkness. A recent study of dysphotopsia completed in our clinic indicates that temporal darkness, or negative dysphotopsia, is the most prevalent symptom today. In this case, the patient detects a black shadow temporally, in the periphery of vision.
I believe this is the result of ring scotoma, a phenomenon frequently experienced by people who wear so-called "cataract glasses." When the central area in our field of vision is magnified, there's a circle of missing information where the magnified view meets the nonmagnified view. Although this ring extends all the way around the visual field, the patient only perceives it as a black region on the temporal side because the nose blocks vision nasally.
This ring of missing information is often associated with a high-plus IOL, and seems to be accentuated when the lens has a high refractive index. Many people don't notice it, but others are immediately bothered by it. It can be severe enough that some people require replacement of the lens.
• Scintillating vision. I believe this is caused by backscatter from the lens combined with microsaccades, which are a normal physiological occurrence. (I've observed that this complaint usually parallels these tiny eye movements.) Of course, you can't change the way patients move their eyes, but this symptom tends to be exacerbated by higher refractive index lenses, so you can decrease the symptom by going to a lower refractive index lens. When dealing with this symptom, the size of the lens doesn't seem to be a factor.
• Arc. This is the patient perceiving the edge of the IOL, which usually only happens at night. It's a common complaint and rarely a serious problem if you tell patients that seeing an occasional arc is normal. It usually resolves over time—especially if the capsule overlaps the IOL edge.
• Flare. This is also a scotopic symptom, produced by coma. Correcting minimal cylinder with night driving glasses will often get rid of it. Making the pupil a little smaller at night will also help. (For more on the latter technique, see "Resolving a Dysphotopsia Crisis".)
• Central flash. As mentioned earlier, this appears to be caused by a peripheral light source reflecting off the internal edge of the IOL. Recent advances in edge design have minimized this symptom.
• Haloes. These may be caused by a multifocal IOL such as the Array (AMO), which produces haloes around lights from each ring transition zone. Most patients will adapt to this, and a smaller scotopic pupil can help in the meantime. Nevertheless, night haloes are the number one reason these IOLs are explanted.
If patients see haloes with a monofocal IOL, it usually indicates the presence of spherical aberration. This shouldn't be a major issue, but if necessary, one of the new aspheric-optic IOLs will help.
How the Patient Reacts
We can't eliminate all unwanted images from every cataract patient's vision. However, we don't necessarily need to, because of the phenomenon of central adaptation. The brain is adept at eliminating unwanted visual input from our perception; the most obvious example is the hole in our visual field where the optic nerve enters the eye. In addition, we get front- and backscatter off our natural lens, our pupils are irregular, and we have blood vessels in our retina that we can't see through. In short, there are a lot of unwanted images in our field of vision, but our brain adapts and eliminates them all. By inserting a new lens, we're just adding a new element to the equation.
This natural process is an important part of resolving unwanted visual images after cataract surgery, and it's a process over which we have some control. The brain is basically an analog computer with a variable gain; in other words, we can increase or decrease how much time and attention we devote to any particular input. This can have a dramatic effect on our perception of that input.
For example, try this experiment: Concentrate as much as you can, all day long, on how your right pantleg touches your kneecap. By the end of the day, you'll find that it hurts. In fact, it can become almost unbearable. This is what happens when you "turn up the gain."
This is also what happens when a patient becomes focused on unwanted images. The images become a huge, painful problem. And the madder the patient gets about the problem, the more he focuses on it. It's a vicious cycle, and it can prevent the brain from adapting and eliminating the images.
Managing Dysphotopsia
Ideally, we'd all like to prevent this problem from occurring in the first place, and there are steps we can take to minimize it. Nevertheless, it's inevitable that some patients will experience unwanted images. In these cases, doing the right things before and after surgery can avoid the greater problem—angry, unhappy patients who fail to adapt.
Here are steps you can take to minimize these symptoms and help patients adapt to whatever unwanted images still occur.
• Create accurate expectations before surgery. The best way to avoid the vicious cycle that undermines the patient's adaptive process is to explain ahead of time what might happen after surgery and how the brain normally manages such issues. Then, the patient won't be surprised if some new, unwanted visual effect accompanies the new lens, and will know to avoid "turning up the gain."
Warning the patient in advance is far easier and more successful than trying to explain after a patient has already started to become upset following surgery. Explaining things at that point may sound like you're making an excuse or dismissing his complaint.
Before surgery, I let patients know that they may experience some unwanted images afterwards. I explain that the images don't mean anything, and that they'll go away over time. Most important, I tell them that it's crucial to keep the gain turned down—if unwanted images occur, don't focus on them. Give your brain time to do its job.
Does this approach really work? I've never had to exchange a lens in one of my own cataract patients—only in upset patients who've come to me from other offices. Some of my patients do complain about unwanted images, but they know the images will go away if they don't focus on them. And the images do go away: Sometimes when patients return later I ask about this. They say, "You're right, the images are gone." The brain has learned to cancel them out.
• Minimize the problem surgically. A number of strategies can help accomplish this:
1. Use the right lens. Certain IOL characteristics appear to correlate with reduced dysphotopsia. Newer lenses have helped by increasing the front curvature of the lens, which minimizes front and back light scattering, and by designing the edge to reduce or eliminate reflections. (See Figure 2) Optic size is important, because a smaller lens may create more edge problems. I wouldn't implant a lens any smaller than 6 mm.
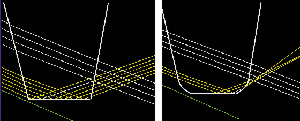 |
| Figure 2. Changing an IOL's square-edge design to a slightly rounded design cuts down the amount of light striking the edge and disperses the remaining reflected rays. This can reduce relative glare intensity as much as 93 percent . |
2. Place the lens carefully. A well-centered, in-the-bag lens prevents unnecessary optical problems.
3. Overlap the capsulorhexis rim over the edge of the lens. The edge of the capsulorhexis will tend to opacify over time, and the opaque overlap will eliminate many symptoms associated with the edge of the IOL. The brain seems to ignore the edge of the capsule, reacting as it does to the edge of the pupil.
This strategy has another major benefit: If you overlap the capsule, you significantly decrease your posterior capsule opacification. Two recent studies, one published by our group, show that overlap of the capsule is more effective at preventing "aftercataract" than switching to an IOL with a truncated edge.1,2
Making a smaller capsulorhexis has some potential downsides. It can be more difficult to access the lens, particularly if you use the Phaco Flip technique. Also, you don't want to risk capsular contracture by making the opening too small. Nevertheless, it's a trade-off: Making the capsulorhexis larger invites more PCO and more dysphotopsia complaints.
To minimize dysphotopsia and PCO, I believe the opening should be roughly 1 mm smaller than the size of the optic, to ensure 360-degree overlap. And use at least a 6-mm optic; every study we've done shows that going below that size dramatically increases overall dysphotopsia and decreases patient satisfaction.
• After surgery, don't take the wrong attitude if a patient complains. The worst thing you can do if a patient complains is to say, "Your result is perfect. Nobody else is complaining. What's your problem?" This virtually guarantees that the patient will "turn up the gain," and fail to adapt to the unwanted images.
Resolving a Dysphotopsia Crisis
A Large-Practice Perspective
Arthur J. Weinstein, MD, chairman of the board at Eye Associates of New Mexico, performs several thousand cataract surgeries each year. He's well-acquainted with the problem of dysphotopsia. "In my practice, this is the single largest area of dissatisfaction associated with uncomplicated, successful cataract surgery," he says. "It's much more frequent than all other complications combined."
According to Dr. Weinstein, only 2 to 3 percent of his patients who receive a square-edge design intraocular lens complain of significant dysphotopsia. However, that translates to 80 or more unhappy patients per year. He performs a lens exchange for 15 to 20 of them each year; the others choose to avoid further surgery in spite of their unhappiness.
"In my experience," he says, "negative dysphotopsia—a dark shadow in the periphery of vision—is much more disconcerting and debilitating than positive dysphotopsia. Some patients describe it as like looking through a keyhole, and some of these people are truly miserable.
"If a patient has negative dysphotopsia and decides he can't tolerate it, we perform a lens exchange. So far, all these patients have done well; symptoms are gone by the next morning, and the patients are extremely relieved. In contrast, I've only had to remove a few lenses because of positive dysphotopsia. The positive symptoms seem to diminish with time, or the patients get more used to them."
In terms of lens characteristics, like Dr. Olson, Dr. Weinstein says he hasn't seen negative dysphotopsia after implantation of a rounded-edge silicone lens. At one point, he says he switched to using all acrylic lenses with square edges for six months, and the incidence of negative dysphotopsia went up dramatically.
"It's hard to say how much of the problem is caused by the material, and how much is due to the edge design," he notes. "I believe acrylic is more prone to dysphotopsia, but I've also had to remove some square-edge silicone lenses. It could be that both factors contribute." In spite of the large number of dysphotopsia cases he's encountered, he says he still hasn't found a way to predict which patients will develop symptoms.
Dr. Weinstein believes the number of patients a surgeon sees may have a profound effect on how well he or she handles this problem. "If you do 400 cases a year, you're only going to see a really unhappy patient with dysphotopsia a few times a year. If you hardly ever see this problem, you may not know what it is or pay much attention to it. You may tell the patient, 'I don't know why you have this. You have to live with it.'
"Cataract surgeons need to know that dysphotopsia is real," he concludes. "They need to listen to the patient and treat this as a legitimate problem." Dr. Weinstein adds that the payoff is significant. "Few patients are happier than those who've had a lens exchange for negative dysphotopsia."
Because I've had many patients come to my office complaining bitterly about unwanted images, I've had ample opportunity to find out what's most helpful when a patient is in full-blown crisis mode. (This experience has also made it clear what not to do.)
• Talk to the patient (and say the right thing). First of all, let the patient know that she's not crazy. That alone will improve matters. Then, try the options listed below. (Just asking the patient to "turn down the gain" may be fruitless at this point. If the patient has been angry and frustrated for several months, it may be impossible for her to do so.)
• Try nighttime pupil constriction. If the problem isn't too severe, and most of the patient's complaints involve nighttime situations such as driving, have the patient try using a few drops of Alphagan P (Allergan) before entering the problematic circumstances. This will cause a little pupil constriction, which is often sufficient to resolve the problem. (Of course, this won't work for problems that occur in daylight conditions.)
Tell these patients to use the drops on an as-needed basis, and let them know that they'll probably only need to use them for a few weeks. This will give the brain time to adapt. At some point they'll forget to use them and realize the problem has disappeared. I find that most patients don't use the drops more than 15 or 20 times.
• Whatever you do, don't open the capsule. Some ophthalmologists, seeing how frustrated the patient is, say, "Well, maybe it's your capsule. Maybe you've got aftercataract. So let's go ahead and do a YAG capsulotomy and see if that will make it better." By the time many patients make it to my office, they've seen four or five different doctors, and someone's tried this approach.
Unfortunately, if the problem truly is dysphotopsia, a capsulotomy won't have any positive effect at all. What it will do is turn a relatively straightforward IOL exchange—which might actually solve the problem—into a very difficult and far riskier procedure. When you try to take the lens out after a YAG capsulotomy, vitreous comes forward. You often can't put the lens back in the capsule because the capsulotomy tears further. The risk of endophthalmitis and retinal detachment increase dramatically.
If the problem is dysphotopsia, don't YAG the capsule.
• Only resort to lens exchange if it really makes sense. First of all, make sure the patient has had enough time to adapt. If six months has transpired, and you've tried Alphagan P drops, then a lens exchange might be worth considering—but only if you can improve on the existing lens situation. Otherwise, switching lenses will be a waste of time.
Factors to consider when deciding whether lens exchange makes sense include:
1. The size of the existing capsulorhexis. If the optic is small and a larger optic will create more overlap of the edge, this could have a positive effect. (If the opening is already large, this isn't an option.)
2. Edge design. If the current IOL doesn't have an up-to-date edge design, this could be part of the problem, and switching to an updated lens would be helpful.
3. Refractive index. If the current lens has a high refractive index, switching to a rounded-edge silicone lens may be curative, particularly if the problem is negative dysphotopsia (seeing the dark region off to the side). If the patient is experiencing scintillating vision on a regular basis, this option is less likely to be curative, but it may be helpful. (Again, if the patient already has a silicone lens with rounded edges and a large capsulorhexis, changing the lens won't help.)
4. Condition of the capsule. If another surgeon has performed a YAG capsulotomy, a lens exchange will involve more risk.
• If all else fails ... For some patients, of course, nothing you do will relieve the symptoms, and IOL exchange may not make sense if the patient already has the most beneficial type and size of IOL. In that case, I talk to the patient again and do my best to help him or her relax and stop thinking about it so much, so the brain has a chance to adapt.
While this isn't guaranteed to produce success, at least patients will understand what's happening and know you don't think they're crazy. And, they'll know you tried your best. That moves the odds of success in your favor.
Dr. Olson is the director of the Moran Eye Center at the University of Utah and professor and chair of the university's Department of Ophthalmology and Visual Sciences.
1. Smith SR, Daynes T, Hinckley M, Wallin TR, Olson R. The effect of lens edge design vs. anterior capsule overlap on posterior capsule opacification. Am J Ophthal 2004;138:521-526.
2. Wejde G, Kugelberg M, Zetterstrom C. Position of anterior capsulorhexis and posterior capsule opacification. Acta Ophthalmol Scand 2004 Oct;82:5:531-4.



