Presentation
A 63-year-old Caucasian female is referred to the Wills Eye Emergency Room after six days of vision loss in her right eye. She complains of pain with right eye movements, and notes she had a headache two weeks ago which has since resolved. She denies diplopia, jaw claudication, weight loss, scalp tenderness, polymyalgia, systemic neurologic symptoms or snoring.
History
The patient had no significant past ocular history or past medical history. Past surgical history was significant for right rotator cuff repair three years ago. Family history was non-contributory. The patient had never smoked tobacco and didn’t use alcohol. The patient wasn’t on any medications.
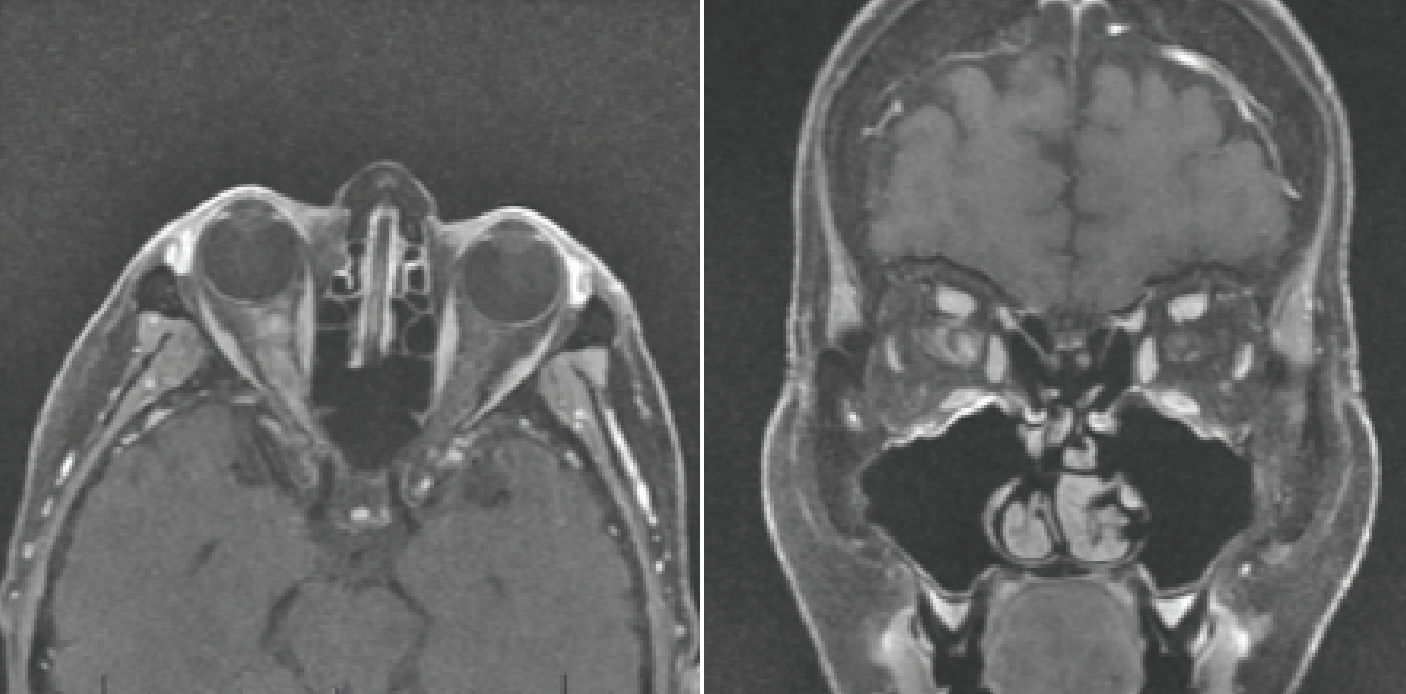 |
| Figure 1. Magnetic resonance imaging revealing long segment hyperintense T1 signal abnormality and enhancement of the intraorbital right optic nerve and optic nerve sheath, characteristic of MOG antibody associated optic neuritis. |
Examination
Ocular examination demonstrated visual acuity of 20/400 in the right eye and 20/30 in the left. A 2+ APD was noted in the right eye without anisocoria. Intraocular pressure was 20 and 19 mmHg in the right and left eyes, respectively. Confrontation visual fields were full in both eyes, but Amsler grid testing revealed a central scotoma in the right eye. Extraocular motility was full bilaterally. Color plates were 4/8 in the right eye, and 8/8 in the left. Anterior segment examination was notable for 1+ nuclear sclerosis bilaterally.
Dilated fundus examination of the right eye demonstrated 360 degrees of blurred disc margins without obscuration of vessels, hemorrhage or pallor. The left optic disc was sharp without edema, pallor or hemorrhage. There was no vitritis noted in either eye.
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
MRI brain and orbits with and without contrast revealed long segment hyperintense T2 signal abnormality and enhancement of the intraorbital right optic nerve (Figure 1). The patient’s symptoms, exam findings and imaging supported the diagnosis of acute optic neuritis of the right optic nerve. The differential diagnosis of this patient included ischemic optic neuropathy, inflammatory, infectious, and neoplastic etiologies. Vascular etiologies include giant cell arteritis, Sjögren’s syndrome. Inflammatory etiologies include multiple sclerosis-associated optic neuritis, neuromyelitis optica and myelin oligodendrocyte glycoprotein (MOG) antibody disease. Infectious etiologies include Bartonella, Lyme, tuberculosis, syphilis, and HSV or VZV.
The lab work-up for targeted vascular, autoimmune, infectious and infiltrative diseases (ESR, CRP, Platelets, ACE, Quant-Gold, Syphilis, Lyme, Bartonella) were negative, and MOG and NMO were pending.
The patient was admitted to Wills Eye Hospital for intravenous, pulse-dose steroids. During admission, her visual acuity improved from 20/400 to 20/30, and her color plates improved from 4/8 to 7/8. She was discharged on an oral prednisone taper. Her MRI C/T spine showed no spinal lesions. She returned to clinic a week later, and her visual acuity and improved to 20/25, and her color plates were 8/8. Optical coherence tomography and B-scan ultrasound images were obtained. OCT demonstrated right-sided optic disc edema with decreased thickening of the retinal nerve fiber layer in the right eye (Figure 2). Her Humphrey visual field testing was normal in the left eye and revealed a small residual paracentral scotoma in the right eye. Her MOG testing returned positive 1:40, and her NMO was negative. She has continued to improve on subsequent follow-up visits without recurrence over four months.
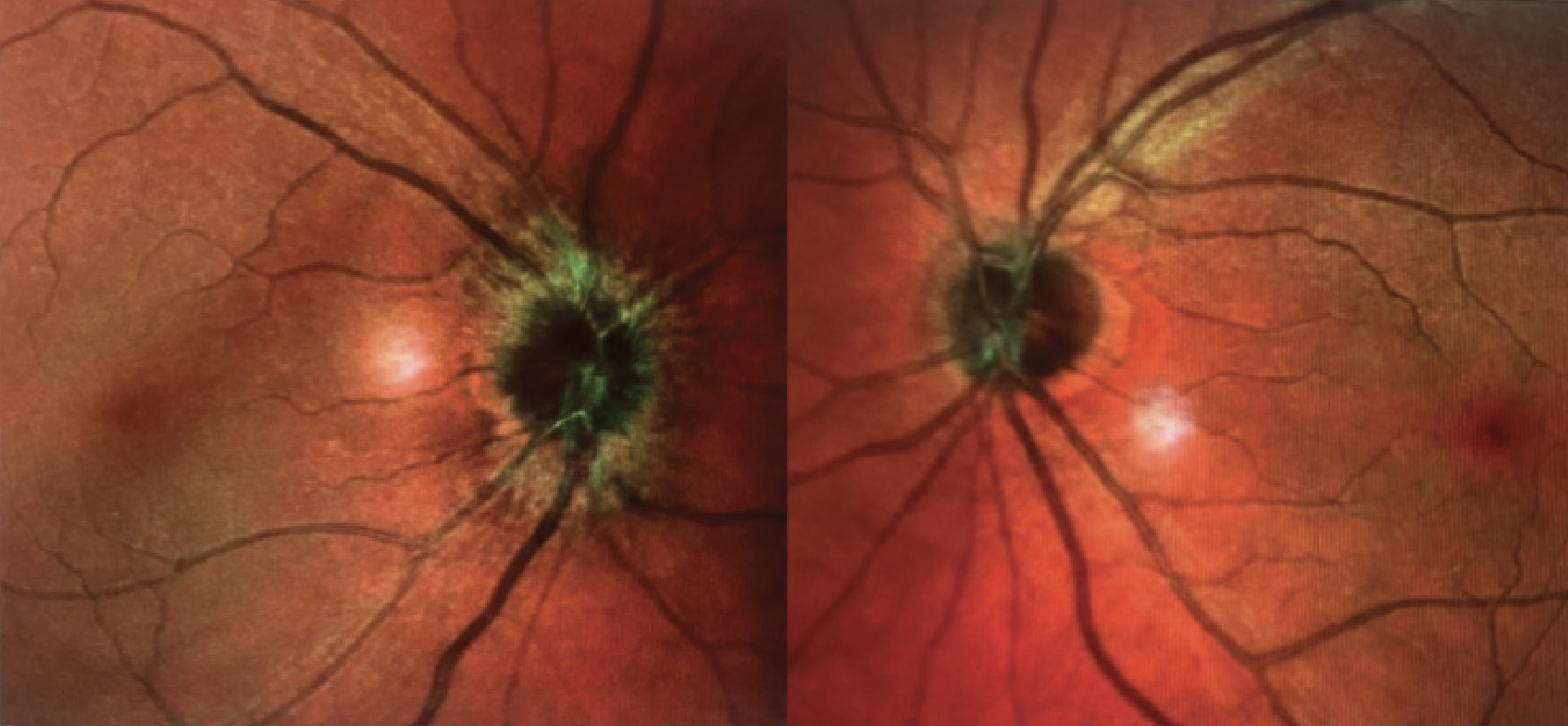 |
| Figure 2. En face multicolor optical coherence tomography revealing optic disc edema of the right eye. |
Discussion
Inflammatory demyelinating optic neuritis can result from three main diseases: multiple sclerosis; NMO; and MOG antibody disease. These diseases are distinguished by the presence of specific autoantibodies in the serum that target different antigens in the central nervous system. Anti-aquaporin-4 (AQP4) antibodies are characteristic of NMO, while anti-MOG antibodies are specific for MOG antibody disease. Anti-AQP4 antibodies have the capacity to induce demyelination by themselves, whereas anti-MOG antibodies require additional factors to cause tissue damage.1 This is one reason why it’s believed that anti-AQP4 positivity is associated with disease activity, while anti-MOG antibody levels persist even after the resolution of the acute phase.2,3
MOG antibody disease manifests as demyelinating lesions in the cerebral hemispheres, brainstem and spinal cord that sometimes resemble those of multiple sclerosis. Optic neuritis in MOG is more likely to affect both eyes simultaneously and present with more profound visual loss than MS-associated optic neuritis. Ophthalmoscopic examination in MOG reveals optic disc swelling more frequently than in MS. Moreover, magnetic resonance imaging in MOG antibody disease shows longer segments of optic nerve enhancement anterior to the optic chiasm, whereas multiple sclerosis typically presents with a short segment of retrobulbar enhancement.4 Despite the more severe initial presentation of optic neuritis in MOG compared to MS, both conditions generally have favorable visual outcomes and respond well to steroids.5 This contrasts with NMO, which is associated with a poorer visual prognosis.6
The patient in this case had several features suggestive of MOG antibody disease. She didn’t match the typical age profile for optic neuritis associated with multiple sclerosis. The patient’s visual acuity was 20/400, which is worse than the usual range for optic neuritis associated with MS. The patient exhibited optic disc swelling, perineural enhancement and a long segment of optic nerve enhancement on MRI. Furthermore, the patient experienced a prodromal headache preceding the onset of optic neuritis, which has been reported in up to half of cases of MOG optic neuritis.7 In cases of optic neuritis that deviate from the common presentation, it is essential to consider the differential diagnosis of MOG or NMO and to monitor visual acuity closely in case plasma exchange is required in addition to high-dose corticosteroid therapy.8,9
1. Höftberger R, Guo Y, Flanagan EP, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathologica 2020;139:5:875-892.
2. Jarius S, Wildemann B, Paul F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clinical and Experimental Immunology 2014;176;2:149-164.
3. Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. Journal of Neuroinflammation 2016;13:1.
4. Wynford-Thomas R, Jacob A, Tomassini V. Neurological update: MOG antibody disease. Journal of Neurology 2019;266:5:1280-1286.
5. Akaishi T, Himori N, Takeshita T, et al. Five-year visual outcomes after optic neuritis in anti-MOG antibody-associated disease. Multiple Sclerosis and Related Disorders 2021;56:103222.
6. Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG related optic neuritis—Prevalence, optical coherence tomography findings, and visual outcomes: A systematic review and meta-analysis. Frontiers in Neurology 2020 Oct 8;11:540156. doi: 10.3389/fneur.2020.540156. eCollection 2020.
7. Asseyer S, Hamblin J, Messina S, et al. Prodromal headache in MOG-antibody positive optic neuritis. Multiple Sclerosis and Related Disorders 2020;40:101965.
8. Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurology - Neuroimmunology Neuroinflammation 2019;6:4:e572.
9. Fu J, Wang Y, Li H, et al. Efficacy of plasma exchange treatment for demyelinating optic neuritis associated with various serum antibodies: A prospective cohort study. Neurology and Therapy 2022;11:2:797-813.



