Although exudative age-related macular degeneration is treatable, treatment efficacy is currently low. Traditional laser photocoagulation not only causes scotomas, but also is associated with persistence or recurrence of treated vessels. Moreover, photocoagulation is not applicable to the majority of patients due to poorly defined neovascularization. Photodynamic therapy with Verteporfin (Visudyne, Novartis) can exert beneficial effects in some AMD patients with choroidal neovascularization, but treatment efficacy appears to be limited to lesions of certain sizes and angiographic constitution, multiple retreatments are typically required, and many patients continue to experience vision loss despite appropriate therapy. For those AMD patients not eligible for PDT, other treatment modalities are required.
Therefore, the development of new and adjuvant therapies, especially the use of antiangiogenic compounds, remains a high priority in the treatment of exudative AMD. Combination therapy for AMD, in which treatments addressing different pathogenic sites are combined to maximize treatment benefit, is a new concept in AMD treatment. This approach may facilitate a synergistic treatment benefit using existing or emerging treatments, therapies that may have only limited benefit when utilized individually. For example, PDT combined with intraocular antiangiogenic corticosteroids is already being researched and utilized in some practices, and this treatment will serve as a model for other combined therapeutic approaches in the future.
CNV Pathogenesis
An understanding of the pathogeneses of CNV is important in combination therapies. Traditionally, RPE aging has been associated with AMD. Senescent RPE can lead to drusen formation and promotes further dysfunction of the remaining RPE. Bruch's membrane, beneath the RPE, can become clogged with debris and may develop crack-like defects. Calcification and fragmentation of Bruch's membrane is thought to facilitate CNV formation in exudative AMD as well as in myopic degeneration and angioid streaks. Macrophages, involved in the initial response to Bruch's membrane injury, may secrete angiogenic growth factors that lead to an angiogenic cascade.
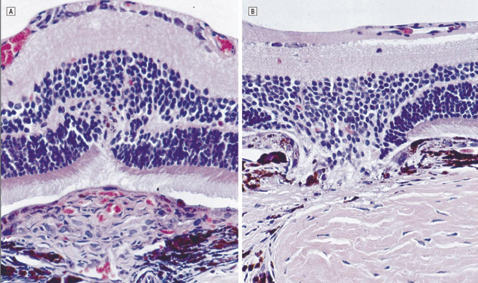 |
| A control eye (left) treated with isotonic sodium chloride solution. Note the fibrovascular proliferations (FVP) arising through the disrupted retinal pigment epithelium and the Bruch's membrane and infiltrating the retina. There are red blood cells seen within luminal structures. Right, a triamcinolone acetonide–treated eye. Note the prominent defects in the RPE and the Bruch's membrane with a striking absence of FVP. The retina is drawn into the defect. Copyrighted 2001, AMA. All Rights reserved. Arch Ophthalmol vol. 119, Mar 2001 |
Macrophages have been found in association with large drusen, breaks in Bruch's membrane, and CNV. Macrophages contain glucocorticoid-binding proteins and secrete pro-inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor (TNF)-alpha, with potent angiogenic properties likely to promote the occurrence and growth of CNV.
Numerous angiogenic growth factors probably are involved in the complex mitotic, morphogenic, and motogenic events that culminate in CNV development. Surgically excised and post-mortem CNV tissue, as well RPE cells, have demonstrated immunoreactivity for various growth factors, including: basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-b), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and platelet derived growth factor (PDGF). Alternatively, pigment epithelium derived growth factor (PEDF) seems to be one of an emerging group of intrinsic antiangiogenic factors.
Matrix metalloproteinases (MMP) are enzymes that degrade the extracellular matrix and are thought to contribute to CNV angiogenesis. Bruch's membrane contains tissue inhibitor of metalloproteinase (TIMP)–3 a tissue inhibitor of metalloproteinases, and eyes with AMD have demonstrated elevated levels of TIMP-3 compared to normal age-matched control eyes. Elevated TIMP-3 is associated with a thickened, abnormal Bruch's membrane that facilitates CNV development.
Antiangiogenic Corticosteroids
Antiangiostatic corticosteroids may exert an effect on CNV through several of these pathways. For example, they may act to inhibit growth factors involved in CNV formation. In particular, prednisolone was recently shown to inhibit VEGF, PDGF and bFGF-induced human RPE cell proliferation in vitro. Also, triamcinolone acetonide downregulates in vitro MMP activation and thus may serve to inhibit CNV. In addition, antiangiogenic corticosteroids also are thought to inhibit macrophages that release angiogenic growth factors. Finally, antiangiogenic corticosteroids can alter extracellular matrix turnover, which further interferes with angiogenesis. In particular, triamcinolone also down-regulates intercellular adhesion molecule-1, which mediates leukocyte adhesion, and transmigration and is expressed by RPE and endothelial cells.
Intravitreous dexamethasone and triamcinolone acetonide injections potently inhibit CNV in primate and rat laser-trauma models. In humans, several pilot studies using intravitreous triamcinolone acetonide suggest some beneficial effect for AMD-related CNV. A larger controlled study reported no significant improvement in vision with a single injection. However, during the first three months after the injection, there was a definite decrease in neovascular growth, suggesting a role for repeated injections or the use of injections in combination with other treatments.
Corticosteroids Combined with PDT
These studies have led investigators to a combination therapeutic approach, in which PDT is combined with intraocular triamcinolone. In one uncontrolled preliminary study, combining 4 mg triamcinolone and PDT, 33 percent of 13 patients with new onset AMD exhibited improvement in visual acuity of at least three lines at six months, although results were much less favorable in the 13 AMD patients previously treated with PDT. In another uncontrolled study of 14 patients in which 11 received one initial combined treatment and three received an additional combined treatment after six months, 7 percent gained ³30 letters, 50 percent maintained stable vision, 14 percent lost 15-29 letters, and 29 percent lost ³30 letters. Overall, mean GLD increased from 2580 (SD 1088) µm to 3946 (SD 1503) and the mean number of PDTs during the first year was 2.57. Side effects were mild: intraocular pressure elevation in 28.5 percent and cataract progression in 50 percent of phakic eyes.
The authors of these preliminary studies conclude that this combination therapy appears to be safe. The potential mechanisms for a possible synergistic effect include the observations that: 1) triamcinolone decreases exudation to facilitate PDT through improved laser penetration and activation; 2) triamcinolone decreases VEGF expression that may be induced after PDT; and 3) triamcinolone decreases fibrosis after PDT treatment. A large prospective trial is currently planned.
Future of Combination Therapy
As noted, PDT combined with intraocular antiangiogenic corticosteroids will serve as a model for other combined therapeutic approaches in the future. The newly emerging antiangiogenic therapies currently in Phase 3 clinical trial will not completely inhibit CNV growth or exudation.
Combining these treatments to address different pathogenic sites may maximize treatment benefit, while minimizing need for retreatment. Macugen, Lucentis, and Retaane are currently in late-phase clinical development and may ultimately be combined in clinical practice with interventions that acutely close CNV, such as PDT (or transpupillary thermotherapy currently undergoing Phase 3 clinical trial). It is also possible that radiation therapy, which may also limit CNV growth, or surgical intervention, such as macular translocation or surgical excision of CNV, may have a role in AMD combination treatment.
The ophthalmic community eagerly looks forward to the time when these treatments and new approaches address this most common cause of legal blindness in the industrialized world.
Dr. Ciulla practices in the Macula-Retina-Vitreous Section at Midwest Eye Institute.
Suggested Reading:
1. Kaven C, Spraul CW, Zavazava N, et al.: Thalidomide and prednisolone inhibit growth factor-induced human retinal pigment epithelium cell proliferation in vitro. Ophthalmologica 2001;215:284-289.
2. Wang YS, Friedrichs U, Eichler W, et al. Inhibitory effects of triamcinolone acetonide on bFGF-induced migration and tube formation in choroidal microvascular endothelial cells. Graefe's Arch Clin Exp Ophthalmol 2002;240:42-48.
3. Penfold PL, Wen L, Madigan MC, et al. Triamcinolone acetonide modulates permeability and intercellular adhesion molecule-1 ICAM-1 expression of the ECV304 cell line: implications for macular degeneration. Clin Exp Immunol 2000;121:458-465.
4. Ciulla TA, Criswell MH, Danis RP, et al. Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants. Br J Ophthalmol. 2003;87:1032-1037.
5. Ciulla TA, Criswell MH, et al.: Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Ophthalmol 2001;119:399-404.
6. Penfold P, Gyory J, Hunyor A, et al.: Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol 1995;23:293-298.
7. Danis RP, Ciulla TA, Pratt LM, et al.: Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 2000;20:244-250.
8. Ranson NT, Danis RP, Ciulla TA, et al. Intravitreal triamcinolone in subfoveal recurrence of choroidal neovascularisation after laser treatment in macular degeneration. Br J Ophthalmol 2002;86:527-529.
9. Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998, 26:277-281.
10. Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF. Intravitreal reinjection of triamcinolone for exudative age-related macular degeneration. Arch Ophthalmol 2004 Feb;122:218-22.
11. Gillies MC, Simpson JM, Luo W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol 2003;121:667-673.
12. Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2003;110:1517-1525.
13. Rechtman E, Danis RP, Pratt LM, Harris A. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age-related macular degeneration. Br J Ophthalmol 2004 Mar;88(3):344-7.