
Presentation
A 27-year-old Caucasian woman presented to an outside emergency room complaining of a left-sided headache and blurry vision in her left eye for two days. Neither ophthalmic evaluation nor imaging studies were performed during the ER evaluation. The patient was diagnosed with a migraine headache and discharged home with Percocet tablets. Seven days later, the patient presented to the Wills Eye ER complaining of worsening headache and vision associated with nausea and vomiting.
Medical History
Past ocular history was unremarkable. Past medical history was significant for hepatitis C, for which the patient had not received treatment. Her surgical history was notable for a cholecystectomy. The patient denied use of any medications. She smoked 10 cigarettes per day, denied alcohol consumption and had a previous history of intravenous drug abuse. Her family history was non-contributory.
Examination
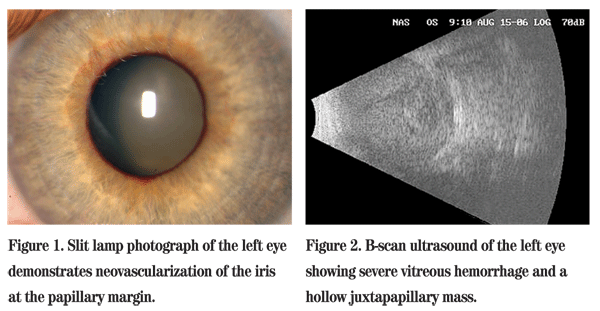
On examination at the Wills ER, her best corrected visual acuity was 20/25 in the right eye and no light perception in the left eye. Visual field exam in the right eye was full to confrontation. The right pupil was round and reactive, and the left pupil was amaurotic. Intraocular pressures by applanation were 14 mmHg in the right eye and 40 mmHg in the left eye. Extraocular muscles had full range of motion in both eyes. Anterior segment exam of the left eye revealed a flat anterior chamber and neovascularization of the iris (See Figure 1). Gonioscopy revealed an open angle in the right eye; the angle in the left eye was closed. Dilated fundus exam was unremarkable in the right eye. Examination of the left eye revealed retina abutting the posterior lens capsule. B-scan ultrasonography was performed and revealed a retinal detachment with subretinal hemorrhage and a questionable mushroom-shaped mass with vascular pulsations (See Figure 2). Transillumination of the globe revealed no shadow defects.
Diagnosis, Workup and Treatment
The mechanisms that can be responsible for angle-closure glaucoma include etiologies causing anterior displacement of the lens-iris diaphragm, contraction of a membrane over the trabecular meshwork, and pupillary block. The differential diagnosis of anterior displacement of the lens-iris diaphragm includes exudative retinal detachment, suprachoroidal hemorrhage (spontaneous or traumatic), choroidal effusion (e.g. uveal effusion syndrome), Vogt-Koyanagi-Harada syndrome, medication-induced anterior displacement, posterior scleritis, or posterior segment tumor such as malignant melanoma, metastatic carcinoma, retinal capillary hemangioma or advanced vasoproliferative tumor. Angle-closure glaucoma can also be secondary to an inflammatory condition (chronic uveitis) or neovascular membrane (e.g., diabetic retinopathy, CRVO, CRAO, ocular ischemic syndrome) that closes the anterior chamber angle. A membrane can also be secondary to epithelial or endothelial downgrowth causing iridocorneal endothelial syndrome or posterior polymorphous membrane dystrophy. 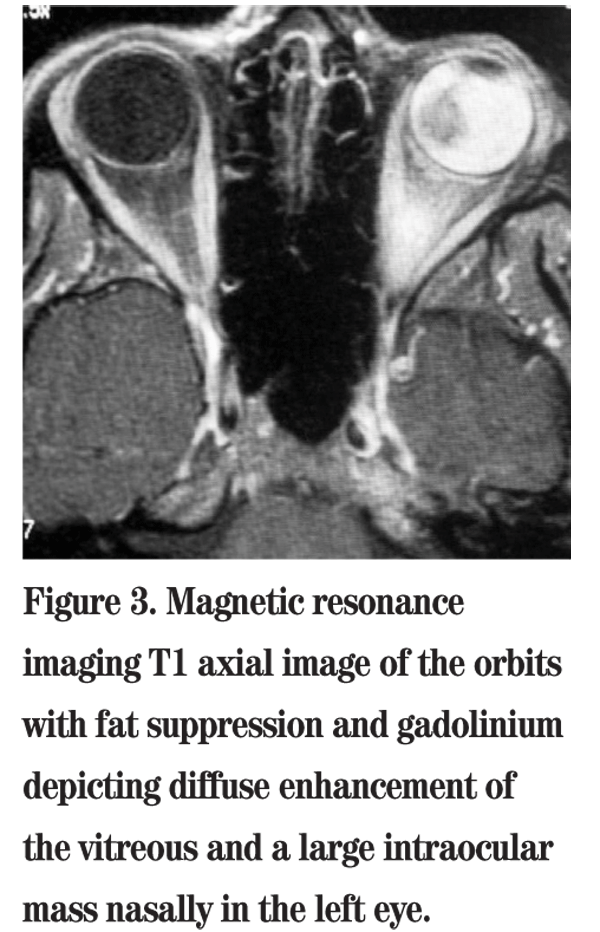
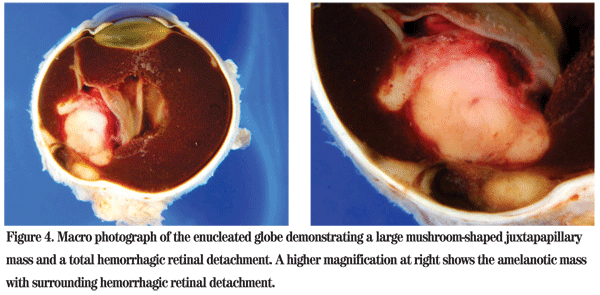
The patient was referred to the Wills Eye Institute Ocular Oncology Service for further evaluation and management. Her examination and imaging were suspicious for a posterior segment mass, and an MRI was ordered which demonstrated an enhancing intraocular mass with no orbital involvement (See Figure 3). Given that the patient had no light perception vision, a total retinal detachment and intractable pain in her left eye, enucleation was performed. Pathology of the globe revealed a large amelanotic mushroom-shaped mass arising from the choroid with an associated total hemorrhagic retinal detachment. Microscopic evaluation showed a large mixed-cell type malignant melanoma of the choroid measuring 11 mm in basal diameter and 10.7 mm in thickness (See Figures 4, 5). Additional features included iris neovascularization and secondary angle-closure glaucoma.
Discussion
Choroidal melanoma is the most common primary ocular malignancy with approximately 2,000 new cases each year in the
Choroidal melanoma typically occurs in the 5th or 6th decade. Sixty-five percent of melanoma patients are over the age of 50 years. Although rarely seen in children, when it does occur, the prognosis is better compared to adults. Choroidal melanoma is more common in Caucasians, and the majority of cases occur in North America and
A number of signs and symptoms lend themselves to a clinical suspicion of choroidal melanoma. Symptoms include blurred vision, visual field defects, flashes, floaters and pain. Findings include a gray/brown or yellow/white mass. The mass can be mushroom-shaped, dome-shaped or diffuse. Subretinal fluid and exudative retinal detachment can be seen. The height of choroidal melanoma is typically >2 mm, thus differentiating it from a choroidal nevus which is classically <2 mm in thickness. Orange pigment, subretinal fluid, vitreous hemorrhage, and extrascleral extension are also features associated with choroidal melanoma. Malignant melanomas of the choroid can undergo necrosis, leading to intraocular inflammation and pain. Additionally, they can lead to secondary glaucoma. Shields and associates reported on 2,704 consecutive eyes with melanoma and found intraocular pressure elevation secondary to the tumor in 5 percent. Causes for the pressure elevation typically included neovascular glaucoma, angle-closure glaucoma from total retinal detachment, and infiltration of the trabecular meshwork by malignant melanoma cells. Malignant uveal melanoma is the most common tumor in adults to result in secondary glaucoma.
The differential for choroidal melanoma includes both pigmented and non-pigmented lesions. Pigmented lesions include choroidal nevus, subretinal hemorrhage from macular and extramacular degeneration, congenital hypertrophy of the retinal pigment epithelium, retinal pigment epithelium hyperplasia and choroidal detachment. Non-pigmented lesions include choroidal hemangioma, metastatic carcinoma, choroidal osteoma, posterior scleritis and lymphoma.
Many evaluation modalities can be utilized when choroidal melanoma is suspected. Transillumination of the globe can show a shadow defect, particularly with ciliary body and peripheral choroidal melanoma. Ultrasonagraphy is also an important tool in characterizing the tumor. A-scan shows low-moderate internal reflectivity while B-scan typically demonstrates a dome-shaped or mushroom-shaped, solid tumor. Fluorescein angiography reveals a characteristic "double" circulation pattern. Finally, MRI can be used to evaluate the tumor"s size and extent. Choroidal melanoma appears bright on T1 and dark on T2 weighted images. 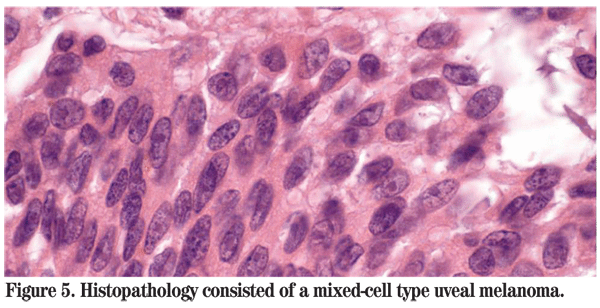
Early detection of choroidal melanoma is of utmost importance. Shields and coworkers coined the mnemonic TFSOM as a tool to aid in differentiating small choroidal melanomas from choroidal nevi. TFSOM (to find small ocular melanoma) is an acronym for the risk factors of Thickness (>2 mm), subretinal Fluid, Symptoms,
For many years, enucleation was the only treatment available for choroidal melanoma. Today, the choice of management options depends on the characteristics of the individual tumor. Transpupillary thermotherapy (TTT) is a technique that uses a diode laser to deliver infrared radiation to the tumor causing tumor cell necrosis. This management option is most efficacious for pigmented tumors <3mm thick and >3 mm from the foveola. As it is maximally effective at the apex of the tumor, it has been shown to be successful when combined with plaque brachytherapy in a technique called sandwich therapy. Radiotherapy such as plaque brachytherapy using iodine-125, ruthenium-106 or other isotopes is useful for tumors <10 mm. The Collaborative Ocular Melanoma Study (COMS) showed that plaque radiotherapy provides local control comparable to enucleation and parallels survival rates equal to enucleation for medium choroidal melanomas. Local resection can be done for anterior tumors, and it is often supplemented with postsurgical adjuvant brachytherapy. Enucleation is recommended for advanced tumors, those with optic nerve invasion, and tumors with secondary glaucoma. Management with exenteration for cases with extrascleral extension is controversial.
The prognosis of a choroidal melanoma is determined by its pathology and chromosomal makeup. Spindle A pathology indicates a five-year survival of 95 percent. Epithelioid pathology has a five-year survival rate of less than 30 percent. Newer information on abnormalities of chromosomes 3, 6 and 8 have been shown to be predictive of mortality. Loss of chromosome 3 (monosomy 3) is associated with aggressive tumors and a poor prognosis for survival. Addition of 8q also implies a poor prognosis, whereas loss of the long arm of chromosome 6 implies a better prognosis.
The authors thank Carol Shields, MD, of the Wills Eye Institute Ocular Oncology Service for her assistance with this case.
Margo CE. The Collaborative Ocular Melanoma Study: An overview. Cancer Control 2004 Sep-Oct;11(5):304-9.
Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004 Oct 15;64(20: 7205-9.
Pesin SR, Katz LJ, Augsburger JJ, Chien AM, Eagle RC Jr. Acute angle-closure glaucoma from spontaneous massive hemorrhagic retinal or choroidal detachment. An updated diagnostic and therapeutic approach. Ophthalmology 1990;97:76-84.
Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol 2002 Jun;13(3):135-41.
Shields CL, Shields JA. Recent developments in the management of choroidal melanoma. Curr Opin Ophthalmol 2004 Jun;15(3):244-51.
Singh AD, Damato B, Howard P, Harbour JW. Uveal melanoma: Genetic aspects. Ophthalmol Clin North Am 2005 Mar;18(1):85-97.



