Etiology & Clinical Challenges
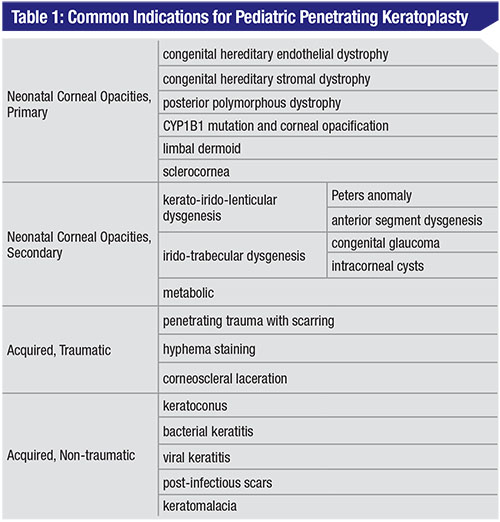
The causes of pediatric corneal opacities vary by age group. In neonates, corneal opacities are likely secondary to primary corneal disease, whereas in older children they are mostly due to acquired traumatic and non-traumatic causes.6 The etiologies are summarized in Table 1.
Obtaining an accurate assessment of the anatomy is one of the most important steps in the preoperative evaluation, as this will aid in diagnosis and prognosis. However, it’s often difficult to obtain an adequate exam in younger patients due to limited cooperation, and an examination under anesthesia may be necessary.7 Surgical interventions can be planned concurrently or at a subsequent evaluation. It’s of the utmost importance to appropriately educate the child’s parents or guardians regarding the frequency of postoperative follow-up with the cornea surgeon, as well as with the pediatric ophthalmologist for amblyopia management, in order to maximize the child’s visual potential.2-4,6
Penetrating Keratoplasty
Traditionally, PKP was the treatment of choice for many corneal opacities. While older articles advised against keratoplasty in infants, recent literature recommends surgical intervention as early as possible to decrease the risk of amblyopia. This is particularly true in bilateral congenital corneal opacities, in which it’s recommended to perform surgery within the first three months of life to allow for better visual rehabilitation.3,4,8,9 In unilateral diseases, the chronicity of the condition, the risks of repeated anesthesia and the burden of prolonged postoperative care should be factored into the decision to proceed with surgery.1,4,7,10 Here are the salient aspects of PKP in these patients:
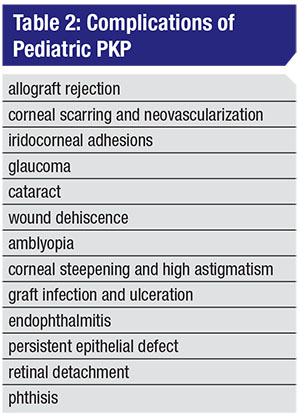
• Intraoperative challenges and postoperative complications. The challenges of PKP stem from the mechanical characteristics of the child’s cornea. Its reduced rigidity makes it prone to collapse intraoperatively, increasing the risk of iris-lens diaphragm prolapse during “open-sky” techniques. The small corneal diameter of the pediatric eye makes the intraoperative process challenging, as well. Other possible complications are summarized in Table 2.4,11–13
Most studies show a higher graft survival rate in patients with acquired opacities (up to an 85-percent one-year survival rate) when compared to the group with congenital opacities (50 to 90 percent).10,11,14,15 In the group of congenital opacities, congenital hereditary endothelial dystrophy seems to carry the best prognosis, with a survival rate of up to 88 percent at one year.14,16-18 It’s suggested that the lack of anterior segment disease and/or corneal neovascularization in this condition leads to a more favorable outcome.16 In contrast, other congenital forms, such as Peters anomaly, present a more variable one-year survival rate, ranging from 49 to 90 percent.12,14,19–21 This wide range is thought to be secondary to the heterogeneity of this disease group. Peters anomaly type 1 has a milder presentation with no lenticular abnormalities and tends to have a high survival rate at one year.4,20 Alternatively, Peters anomaly type 2 diffusely involves the lens and anterior chamber with a higher rate of associated ocular comorbidities such as glaucoma, which usually leads to a poor PKP outcome.22 Lastly, the highest risk of failure is seen in congenital glaucoma and sclerocornea, with only a 50-percent chance of success.5,17
Graft failure can occur at any point during the postoperative period, but happens more frequently within the first year post-transplant.4,5 It can be due to a variety of causes, with the most common being rejection and infection. Children tend to have a more severe postoperative inflammatory response than adults, which increases the risks of synechiae formation and graft rejection.1,3,4,9 The prolonged use of postoperative topical
 |
Graft survival isn’t only affected by the type of opacity, but also by other intraoperative and postoperative variables. Several studies have shown that when PKP is combined with other procedures such as lensectomy/vitrectomy, the majority of children will eventually present with graft failure.11,21 This highlights the high risk of failure in more severe cases needing multiple interventions at the time of PKP.1,4,11,14 One study found that corneal neovascularization also suggests a lower chance of graft survival.13 Additionally, glaucoma is an important consideration after PKPs. Studies have shown that its occurrence before or after transplant is a powerful indicator of graft failure, regardless of the status of IOP control. This is thought to be due to the endothelial cell damage that occurs with high IOP, predisposing the graft to rejection and failure.2,14,23
• Visual outcome. While most patients’ visual acuities improve postoperatively, only 20 to 50 percent of pediatric patients undergoing PKP reach vision better than 20/80, despite good anatomic outcomes.1,7 Earlier timing of surgery hasn’t been shown to improve visual outcome; therefore, the timing of surgery should be considered on a case-by-case basis, taking into account the depth of amblyopia and the severity of the disease.4,14,16 This highlights the importance of amblyopia therapy in the visual rehabilitation after a clear visual axis is achieved by PKP.4,11 This process involves refractive correction with early contact lens fitting and suture removal as soon as a month postoperatively.4,7,10 Since children are prone to accidental trauma, the eye may need to be shielded and the patients may need to wear protective polycarbonate spectacles.1
Anterior Lamellar Keratoplasty
ALK is a surgical procedure that involves the removal of the corneal stroma down to Descemet’s membrane. It’s indicated in corneal pathology that involves the anterior corneal layers but spares the endothelium.25 Since the most common cause of graft failure after PKP is endothelial rejection, avoiding replacement of the endothelium dramatically improves graft survival.5 Also, since the structural integrity of the eye is preserved in ALK, there’s less risk of devastating complications such as expulsive hemorrhage or graft dehiscence.26
Recently, new advances in surgical instrumentation and techniques have managed to optimize the technique of deep anterior lamellar keratoplasty and have shown visual outcomes comparable to PKP but with a higher safety profile.26 The different techniques in DALK include manual dissection, big-bubble technique and femtosecond-assisted anterior lamellar keratoplasty.26-31 Manual dissection is the most basic technique; it involves trephination of the anterior stroma and its dissection from deeper layers using a crescent blade. In contrast, newer techniques involve using an air bubble injection into the deep stroma after trephination to create a plane through which to dissect.26-29 Recent studies have looked at the use of intraoperative optical coherence tomography to guide DALK surgery, which would likely decrease the risk of perforation and increase the success rate.31
In the past decade, the introduction of the femtosecond laser has revolutionized corneal and refractive surgery. This technology, in conjunction with advances in corneal imaging, has allowed for the evolution of a new technique in ALK: femtosecond laser- assisted lamellar keratoplasty.27,30 In FALK, anterior-segment OCT is used to determine the depth of the pathology and the lamellar cut in order to excise only the pathological tissue. The surgeon uses the laser to cut an identical donor corneal lenticule and uses it to replace the excised tissue, creating a smoother interface and yielding better surgical reproducibility. In cases where the corneal pathology spares a residual bed of 300 µm, the surgeon can place the donor corneal lenticule without a suture.27,30 Despite the risks of corneal haze and residual corneal scars, the advantages of FALK remain its sutureless technique and rapid visual rehabilitation, with patients demonstrating an average gain of 2.5 lines in best-corrected vision postoperatively.27,30
• Considerations and outcomes. ALK is an excellent surgical option for children with anterior corneal pathology and a healthy endothelium. The literature has shown a dramatically decreased rate of graft rejection when compared to traditional PKP, with up to a 90 percent graft survival rate.28 Nevertheless, this technique is challenging in younger eyes due to the elastic biomechanics of the cornea, which increase the risk of intraoperative corneal perforation and prolonged surgical time. These operative challenges, as well as the steep learning curve, have affected the popularity of ALK among corneal surgeons. Reported complications are similar to those found in adults, but occur with a higher frequency in children. Early postoperative complications are mainly micro-perforations and a double anterior chamber. Later complications involve graft dehiscence, astigmatism, loose sutures, keratitis, suture infection, graft-host interface haze, interface neovascularization and epithelial downgrowth.26,27,29,30
DSAEK
Endothelial keratoplasty in children was first reported in 2008. This technique involves scoring and removing the recipient Descemet’s membrane, and injecting a donor membrane with its underlying healthy endothelium. An anterior chamber air bubble is then used to keep the graft in place without the need for sutures. In children, scoring of Descemet’s may prove to be difficult, and some surgeons are avoiding this step by injecting the graft directly on top of the host’s membrane, a technique called non-Descemet Stripping Endothelial Keratoplasty.32 Current indications for DSAEK include: pseudophakic bullous edema; Peters anomaly; Descemet’s membrane breaks induced by forceps delivery; posterior polymorphous dystrophy; buphthalmos due to congenital glaucoma; failed corneal grafts; and congenital hereditary endothelial dystrophy.33-38
• Considerations and outcomes. DSAEK is gaining more attention because it solves some of the problems with PKPs. It allows for a faster postoperative recovery, with earlier initiation of amblyopia treatment.32
Additionally, DSAEK employs smaller, 4-mm wounds, which decreases the risks associated with open-sky techniques and also decreases the risk of infection. Suture-related issues are also minimized. Importantly, postoperative refractive errors tend to be less than 3 D of astigmatism, which allows for a faster visual rehabilitation after surgery.32,35,39,40
Nevertheless,
 |
Keratoprosthesis
Keratoprosthesis is a procedure in which an artificial cornea is assembled with a donor corneal graft and sutured to the recipient cornea, providing a clear visual axis in a cornea that’s otherwise opaque.5 The first keratoprosthesis in an infant was performed in 2006 in a patient with Peters anomaly and congenital glaucoma who failed PKP.42 Morbidity rates due to this procedure remain high; therefore it’s currently only recommended in high-risk cases of failing PKP with risk of complete corneal blindness.42 The main complications include retroprosthetic membranes, glaucoma, infection and endophthalmitis.43 Keratoprosthesis remains a high-risk procedure, but its use may be beneficial as a way to prevent deep amblyopia from corneal blindness, until the patient is old enough to undergo a permanent graft.5,43
Though surveying the various options for pediatric transplants and their attendant advantages and drawbacks can be daunting, we hope this review helps to make the picture a little bit clearer. REVIEW
Ms. Koiak is a medical student at the University of Miami’s Miller School of Medicine. Dr. Osigian is an instructor at the University’s Bascom Palmer Eye Institute, and Drs. Shousha and Cavuoto are both assistant professors of clinical ophthalmology at Bascom Palmer.
1. O’Hara M a, Mannis MJ. Pediatric penetrating keratoplasty. Int Ophthalmol Clin 2013;53:2:59–70.
2. Huang C, O’Hara M, Mannis MJ. Primary pediatric keratoplasty: indications and outcomes. Cornea 2009;28:9:1003–1008.
3. Vanathi M, Panda A, Vengayil S, Chaudhuri Z, Dada T. Pediatric Keratoplasty. Surv Ophthalmol 2009;54:2:245–271.
4. Lee, Olivia, Lenhart, Phoebe and Stulting D. Pediatric Penetrating Keratoplasty. I: Cornea. Bd Chapter 12. ; 2016:1455.
5. Hutcheson K a. Application of new ophthalmic technology in the pediatric patient. Curr Opin Ophthalmol 2007;18:5:384–391.
6. Nischal KK. A new approach to the classification of neonatal corneal opacities. Curr Opin Ophthalmol 2012;23:344–354.
7. Hwang DG, Hwang PH. Pediatric Penetrating Keratoplasty. Semin Ophthalmol 1991;6:4:212–218.
8. Comer RM, Daya SM, O’Keefe M. Penetrating keratoplasty in infants. J Am Assoc Pediatr Ophthalmol Strabismus. 2001;5:5:285–290.
9. Reidy JJ. Penetrating keratoplasty in infancy and early childhood. Curr Opin Ophthalmol 2001;12:4:258–261.
10. Patel HY, Ormonde S, Brookes NH, Moffatt LS, McGhee CNJ. The indications and outcome of paediatric corneal transplantation in New Zealand: 1991-2003. Br J Ophthalmol 2005;89:4:404–408.
11. Dana M-R, Moyes AL, Gomes JAP. The indications for and outcomes in pediatric Keratoplasty: A multicenter study. Ophthalmology 1995;102:8:1129–1138.
12. Dana MR, Schaumberg DA, Moyes AL, mfl. Corneal transplantation in children with Peters anomaly and mesenchymal dysgeneses. Ophthalmology 1997;104:10:1580–1586.
13. Yang LL, Lambert SR, Lynn MJ, Stulting RD. Long-term results of corneal graft survival in infants and children with Peters anomaly. Ophthalmology 1999;106:4:833–848.
14. Al-Ghamdi A, Al-Rajhi A, Wagoner MD. Primary pediatric keratoplasty: Indications, graft survival, and visual outcome. J Am Assoc Pediatr Ophthalmol Strabismus 2007;11:1:41–47.
15. Stulting RD, Sumers KD, Cavanagh HD, Waring GO, Gammon JA. Penetrating Keratoplasty in Children. Ophthalmology 1984;91:10:1222–1230.
16. Javadi MA, Baradaran-Rafii AR, Zamani M, mfl. Penetrating keratoplasty in young children with congenital hereditary endothelial dystrophy. Cornea 2003;22:5:420–423.
17. Michaeli A, Markovich A, Rootman DS. Corneal transplants for the treatment of congenital corneal opacities. J Pediatr Ophthalmol Strabismus 2005;42:1:34–44.
18. Schaumberg D a, Moyes a L, Gomes J a, Dana MR. Corneal transplantation in young children with congenital hereditary endothelial dystrophy. Multicenter pediatric keratoplasty study. Am J Ophthalmol. 1999;127:4:373–378.
19. Frueh BE, Brown SI. Transplantation of congenitally opaque corneas. Br J Ophthalmol 1997;81:12:1064–1069.
20. Zaidman GW, Flanagan JK, Furey CC. Long-term visual prognosis in children after corneal transplant surgery for Peters anomaly type 1. Am J Ophthalmol 2007;144:1:104–108.
21. Rao K V, Fernandes M, Gangopadhyay N, Vemuganti GK, Krishnaiah S, Sangwan VS. Outcome of penetrating keratoplasty for Peters anomaly. Cornea 2008;27:7:749–753.
22. Bhandari R, Ferri S, Whittaker B, Liu M, Lazzaro DR. Peters anomaly: Review of the literature. Cornea 2011;30:8:939–944.
23. Gollamudi SR, Traboulsi EI, Chamon W, Stark WJ, Maumenee IH. Visual outcome after surgery for Peters anomaly. Ophthalmic Genet 1994;15:1:31–5.
24. Cosar CB, Laibson PR, Cohen EJ, Rapuano CJ. Topical cyclosporine in pediatric keratoplasty. Eye Contact Lens 2003;29:2:103–107.
25. Buzzonetti L, Petrocelli G, Valente P. Big-bubble deep anterior lamellar keratoplasty assisted by femtosecond laser in children. Cornea 2012;31:9:1083–1086.
26. Ashar JN, Pahuja S, Ramappa M, Vaddavalli PK, Chaurasia S, Garg P. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol 2013;155:3:570–574.
27. Shousha MA, Yoo SH, Kymionis GD, mfl. Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology 2011;118:2:315–323.
28. Arora R, Jain P, Jain P, Manudhane A, Goyal J. Results of deep anterior lamellar keratoplasty for advanced keratoconus in children less than 18 years. Am J Ophthalmol 2016;162:191–198.
29. Harding SA, Nischal KK, Upponi-Patil A, Fowler DJ. Indications and outcomes of deep anterior lamellar keratoplasty in children. Ophthalmology 2010;117:11:2191–2195.
30. Yoo S, Goldman D, Karp CL, Brien TPO, Culbertson WW, Alfonso EC. Femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology 2008;115:8:1303–1308.
31. Cabot F, Vardhaman P, Marco Ruggeri, et al. High-resolution optical coherence tomography-guided donor tissue preparation for descemet membrane endothelial keratoplasty using the reverse big bubble technique. Cornea 2014;33:428–431.
32. Anwar HM, El-Danasoury A. Endothelial keratoplasty in children. Curr Opin Ophthalmol 2014;25:4:340–346.
33. Busin M. Descemet-stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy. Arch Ophthalmol 2011;129:9:1140.
34. Mittal V, Mittal R. Challenges in pediatric endothelial keratoplasty. Indian J Ophthalmol 2014;62:2:251–254.
35. Hashemi H, Ghaffari R, Mohebi M. Posterior lamellar keratoplasty (DSAEK) in Peters anomaly. Cornea 2012;31:10:1201–1205.
36. Jeng BH, Marcotty A, Traboulsi EI. Descemet stripping automated endothelial keratoplasty in a 2-year-old child. J AAPOS 2008;12:3:317-8.
37. Kymionis GD, Kankariya VP, Diakonis VF, Karavitaki AE, Siganos CS, Pallikaris IG. Descemet stripping automated endothelial keratoplasty in a child after failed penetrating keratoplasty. J AAPOS 2012;16:1:95-6.
38. Pineda R, Jain V, Shome D, Hunter DC, Natarajan S. Descemet’s stripping endothelial keratoplasty: Is it an option for congenital hereditary endothelial dystrophy? Int Ophthalmol 2010;30:3:307–310.
39. Fernandez MM, Buckley EG, Afshari NA. Descemet stripping automated endothelial keratoplasty in a child. J AAPOS 2008;12:3:314-6.
40. Goshe JM, Li JY, Terry MA. Successful Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy in a pediatric patient. Int Ophthalmol 2012;32:1:61-6.
41. Lenhart PD, Evans CT, Beck AD, Lee WB. Visual outcome after Descemet’s stripping automated endothelial keratoplasty in an 8-month-old with congenital hereditary endothelial dystrophy. J Am Assoc Pediatr Ophthalmol Strabismus 2013;17:6:637–639.
42. P. Botelho. Keratoprosthesis in high-risk pediatric corneal transplantation: First two cases. Arch Ophthalmol 2006;124:1356–1357.
43. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric Keratoprosthesis. Ophthalmology 2007;114:5:989–994.



