For many years, surgeons have noted the potential advantages of a liquid sealant that could be placed directly onto ocular wounds following surgery. Such a product could potentially protect the eye from leakage, increase surgical efficiency, reduce or eliminate the possibility of endophthalmitis and eliminate the need for sutures. Over the years, a number of products have been used off-label for this purpose, but recently companies have begun producing hydrogel-based materials specifically designed for use on the eye. The synthetic dendritic polymer OcuSeal, for example, received the CE Mark in 2008.
This year a new synthetic hydrogel product, the I-ZIP adherent ocular bandage from I-Therapeutix, received the CE Mark; it has now begun testing in the
Richard Packard, an ophthalmologist in practice at the Prince Charles Eye Unit,
"These cases allowed us to get the feel of the product," he explains. "We got to see what the I-ZIP bandage looks like, how easy it is to mix up and apply, and so forth. It was very simple to use, and it worked extremely well. Two separate elements come in a special kind of syringe; you squeeze out the contents, give it a gentle mix, and then you have about 20 seconds to apply it before it sets. You paint it onto the wound using the tip of a little plastic applicator and it solidifies within a few seconds. We didn't have any problem applying it, and it stayed where we put it.
"Once it's on the eye, the patient has no sensation of it whatsoever," he continues. "There's no impact on tearing that we can detect. Because it's a water-soluble hydrogel, it gradually dissolves; we found that it was gone within a week. It protected the wound during the first few days after surgery when the wound was most vulnerable.
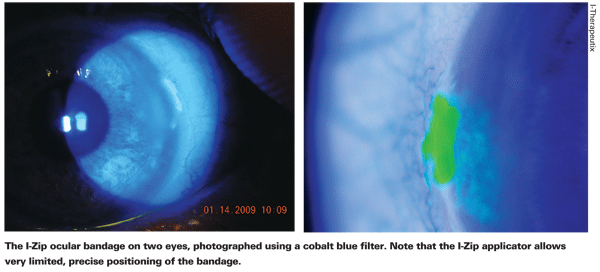
"I think if you're going to use a sealant, this is the sort of thing you want," he says. "Most of the other options for this purpose that we've had in the past are acrylates, which cause tissue shrinkage. This has no effect like that whatsoever—it's much better."
Mr. Packard notes that the I-ZIP bandage could have numerous other potential uses.
"You could use it to do temporary sealing of the tear drainage apparatus to see whether a punctal plug might help someone with dry eye," he says. "Perhaps it could be used to deliver a drug, by combining it with the hydrogel so that the medication is gradually released as the substance dissolves."
Reviewing the Alternatives
Terry Kim, MD, associate professor of ophthalmology and associate director of the Corneal and External Disease Service and Refractive Surgery Service at Duke University School of Medicine in
Dr. Kim notes that previous off-label-use products had some serious disadvantages.
"Cyanoacrylate is a linear polymer that's poorly tolerated on the eye," he notes. "It usually forms a hard, brittle, opaque substance that causes foreign body sensation and may necessitate a bandage contact lens to make it tolerable. And the by-products of cyanoacrylate—such as formaldehyde and cyanide—are toxic to the lens and retina.
There's no question we're in need of an adhesive sealant that's specifically designed for ophthalmic use.
"The OcuSeal product has a brush applicator system that allows the surgeon to apply it in a very thin film on the eye," he continues. "You break the seal and mix the components; then it comes out through the brush applicator and forms a hydrogel within 30 seconds. I recently presented some studies at this year's meeting of the American Society of Cataract and Refractive Surgery showing that the material may possess microbial barrier properties as well."
Cost vs. Risk
Mr. Packard notes that cost will be a major factor in how widely a product like this is adopted. (Although the I-ZIP bandage has received the CE mark, it is not available for purchase in the
"It's unlikely that a surgeon would use a product like this routinely unless it was very inexpensive," he says. "That's because infection is a very rare occurrence in cataract surgery. In our facility here in
Mr. Packard says he's looking forward to the clinical trial. "In the upcoming trial we'll investigate the I-ZIP bandage much more thoroughly, including using anterior segment OCT to see exactly how the bandage affects the wound," he says. "Although we're not using it routinely at this point, it's a very good idea. It may be that it will eventually become a tool that everybody uses."
The I-ZIP bandage is currently under an investigational device exemption and the subject of a multicenter clinical study aimed at achieving U.S. Food & Drug Administration approval.
Mr. Packard and Dr. Kim have no financial interest in either of the products mentioned.
1. Hovanesian J. Cataract wound closure with a polymerizing liquid hydrogel ocular bandage. J Cataract Refract Surg. 2009;35:5:912-6.



