Carotid-cavernous fistula is a relatively rare condition that results from an abnormal communication between the carotid artery and the cavernous sinus.
This article discusses the etiology, diagnosis and treatment of CCF in a typical case (See below), and the latest treatments for what may be its most significant ophthalmic complication, central retinal vein occlusion.
Carotid-Cavernous Fistula
A carotid-cavernous fistula (CCF) is an abnormal connection between the carotid artery (or its branches) and the cavernous sinus. This vascular abnormality is possible because of the unique anatomy of the cavernous sinus. The cavernous sinus is the only site in the body where an artery travels completely through a venous structure. The intracavernous portion of each carotid artery is approximately 2 cm in length and is partially or completely surrounded by venous blood.1 A direct connection between artery and vein introduces high arterial pressure into the normally low-pressure cavernous sinus. The high pressure may reverse blood flow within the superior ophthalmic vein and introduce venous congestion within the orbit.
The typical presenting symptoms of a CCF include headache, eye pain, ptosis and diplopia (from cranial nerve palsies). Frequently, the patient may perceive a "whooshing" or whistling sound in the head. The clinical signs of a CCF include proptosis (which may be pulsatile), arterialization of the conjunctival vessels, elevated intraocular pressure, a cranial bruit and blood in Schlemm's canal upon gonioscopy.
Diagnostic imaging of patients with a CCF will commonly reveal enlargement of the extraocular muscles and a dilated superior ophthalmic vein.
Dural Carotid-Cavernous Fistulas
One subset of carotid-cavernous fistulas is dural venous fistulas. In this type of fistula, also referred to as an arteriovenous shunt, arterial blood passes indirectly through intervening small branches of the external or internal carotid artery before joining the cavernous sinus venous blood. Most branches of the major arteries are located near the meningeal dura mater and, therefore, these fistulas have been termed "dural shunts."1
Dural carotid-cavernous fistulas (DCCF) are vascular abnormalities that are thought to occur as a result of idiopathic thrombosis of small veins within the walls of the cavernous sinus. This blockage in venous blood flow is thought to stimulate arterial collaterals to develop from adjacent arteries. If the collaterals anastomose with the cavernous sinus, a carotid-cavernous fistula results.1Some believe that dural fistulas may be congenital in nature, but may not manifest clinically until later in life.1
The DCCF is considered "low flow" in its circulation of blood. This is in contrast to a direct anastomosis between the intracavernous portion of the internal carotid artery and the cavernous sinus, which is considered "high flow." The clinical findings of a low-flow fistula are less dramatic than those of classic high-flow CCFs. While high-flow fistulas have been linked to severe head trauma, low-flow fistulas frequently occur spontaneously.
Dural venous shunts occur most commonly in elderly women. Arthur S. Grove Jr., MD, reported 10 cases of dural AV shunts and nine of these patients were women.1
The long-term visual prognosis of dural sinus fistulas is very good. This is because many dural sinus fistulas remain stable, while others may even close spontaneously.
DCCF Treatment
Although most dural fistulas are not life-threatening, some may cause severe ocular morbidity. Indications for treating the vascular abnormality include progressive ophthalmoplegia, progressive proptosis with exposure, uncontrolled elevation of intraocular pressure, retinopathy or optic neuropathy, and abnormal cortical venous drainage noted on MRI.2
The treatment of dural carotid-cavernous fistulas may require medical and surgical modalities. Medical treatment is used primarily to control intraocular pressure. Surgical treatment options primarily include percutaneous embolization of spontaneous DCCFs. This may be performed from a transarterial or transvenous approach.3,4 The transvenous approach accesses the superior ophthalmic vein cutaneously. Platinum coils, detachable balloons and liquid acrylic agents have been used for embolization.
DCCF Complications
Although most patients with DCCFs eventually have resolution of their clinical abnormalities without treatment, some patients may experience severe visual complications.1,5 The diversion of arterialized blood into the venous system may compromise blood flow to many different parts of the eye, including the retina and optic nerve. This may result in retinal ischemia or ischemic optic neuropathy.
In addition, diversion of arterial blood directly into the venous system may lead to venous congestion and obstruction within the orbit. Five of the 10 patients with dural AV shunts that were reported by Dr. Grove presented with retinal vascular congestion or retinopathy with hemorrhages. Two of these patients had long-term poor vision from vascular abnormalities.1 Venous outflow obstruction may also lead to central retinal vein occlusion.
The association of carotid-cavernous fistulas with CRVO has frequently been reported in the literature.5-7 In a case presented by Stephen C. Pollock, MD, and Neil R. Miller, MD, an elderly woman with a spontaneous carotid-cavernous fistula developed CRVO when the clinical symptoms and signs of the patient were otherwise improving. The authors of that report also mentioned another patient they had examined with a DCCF and secondary glaucoma, who also developed CRVO.5 Drs. Pollock and Miller emphasized the importance of informing patients with this condition about the possibility of severe visual loss if the fistula is left untreated. They also emphasized that the intraocular pressure should be controlled.5 Isabelle Brunette, MD, and Dan Boghen, MD, reported a patient with a spontaneous carotid-cavernous fistula that subsequently developed CRVO, and suggested that this complication of a DCCF may be more common than previously thought.6 In fact, these authors believed that CRVO might be the main cause of visual loss in patients with a DCCF.6
Central Retinal Vein Occlusion
CRVO is thought to arise from thrombosis of the central retinal vein at the level of, or posterior to, the lamina cribrosa. Venous occlusion raises the venous pressure, slows arterial blood flow and leads to retinal hypoxia and death. This most commonly occurs in patients over the age of 50 and is bilateral in about 5 to 10 percent.
The incidence of retinal vein occlusion (central or branch) increases with advancing age and with the presence of glaucoma.8,9 The Blue Mountain Eye Study evaluated 3,654 patients, 49 years old and older, and found evidence of any retinal vein occlusion in 59 (1.6 percent) and evidence of CRVO in 15 (0.4 percent).9 The Beaver Dam Eye Study followed 2,119 patients, 43 years old or older, for 15 years and found that the cumulative incidence of CRVO during that time period was 0.5 percent.10
Presentation
The most common initial complaint of patients with CRVO is decreased visual acuity. This loss of vision may be severe. In the Blue Mountain Eye Study, patients with CRVO had the sharpest drop in visual acuity compared to other types of vein occlusions, with a visual acuity of <20/200 in 60 percent of patients.9
Clinical Appearance
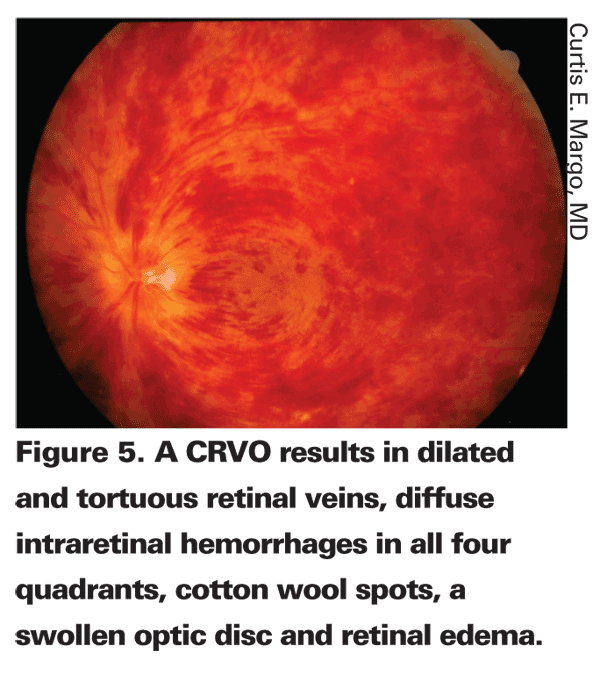
The diagnosis of CRVO is based primarily on the fundus examination. A CRVO results in dilated and tortuous retinal veins, diffuse intraretinal hemorrhages in all four quadrants, cotton wool spots, a swollen optic disc and retinal edema (See Figure 5). These findings are typically unilateral.
Imaging
Fluorescein angiography of CRVO typically demonstrates prolonged retinal circulation time, with variable macular and optic nerve edema. In severe cases, there is diffuse capillary nonperfusion.
Classically, CRVO has been classified into two major types: non-ischemic and ischemic. A non-ischemic CRVO, also referred to as venous stasis retinopathy, is typically mild. It is believed that the non-ischemic type is more common, comprising about 75 percent of all CRVOs. The visual acuity may remain good, and mild visual field changes may occur. However, about 30 percent of patients with a non-ischemic CRVO may progress into an ischemic CRVO within three years.
The ischemic form of CRVO, also known as hemorrhagic retinopathy, requires at least 10 disc diameters of retinal capillary nonperfusion by FA. This severe condition is usually associated with poor vision, an afferent pupillary defect and a central scotoma. Approximately 10 percent of eyes with an ischemic CRVO achieve vision better than 20/400.
Differentiation between ischemic and non-ischemic CRVO may be difficult. CRVO that is indeterminate between ischemic and non-ischemic upon presentation is possible, but more than 80 percent of the indeterminate type progress to the severe, ischemic form.11
Additional Testing
Ancillary diagnostic tests such as visual field testing and electroretinography may be useful in determining whether or not a CRVO is ischemic. A decreased bright-flash, dark-adapted b wave:a-wave amplitude ratio may indicate more severe retinal ischemia, however, this test is not particularly specific for identifying ischemic CRVOs.
Risk Factors
A large, multicenter review of patients with CRVO found that hypertension, diabetes and open-angle glaucoma increased the risk to a patient for developing CRVO.12 The risk of developing CRVO decreased with increasing levels of physical activity and increasing levels of alcohol consumption. This study also found that the risk in women decreased with the use of postmenopausal estrogens and increased with higher erythrocyte sedimentation rates.12
The Blue Mountain Eye Study found that a history of a systemic vascular occlusive event, such as a stroke or myocardial infarction, significantly increased the risk of either a central or branch retinal vein occlusion.9
Several systemic illnesses have also been linked to CRVO. These include blood dyscrasias, such as polycythemia vera, dysproteinemias and vasculitis. Many causes of hypercoagulability of the blood have also been implicated as potential etiologies for CRVOs, such as hyperhomocysteinemia and protein S or C deficiencies.
Treatment
Management of patients with CRVO initially consists of treatment of any systemic medical illnesses that may be coincident with the eye pathology, such as hypertension, hypercholesterolemia and diabetes.
Secondary management involves treating the ocular complications of CRVO that may contribute to visual loss. These include macular edema and glaucoma.
Macular Edema
Macular edema may develop at the same time as the clinical appearance of CRVO or develop subsequently to the vascular event. The diagnosis of macular edema may be made using clinical examination, fluorescein angiography or ocular coherence tomography. The treatment modalities for macular edema include laser photocoagulation and intravitreal injections.
• Laser photocoagulation. The Central Vein Occlusion Study (CVOS) group evaluated focal macular grid pattern photocoagulation for the treatment of macular edema. Although the grid laser clearly reduced angiographic evidence of macular edema, the authors concluded that grid laser yielded no benefit in improving visual acuity.13 The study data, however, revealed a trend suggesting that grid laser might improve visual acuity in younger patients with macular edema as a result of CRVO.13 Due to the results of this trial, observation became a potential way to manage macular edema from CRVO and continues to be utilized by many clinicians in certain clinical scenarios.
• Anti-VEGF agents. The intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents to treat macular edema in patients with CRVO has also been shown to be successful. Philip J. Rosenfeld, MD, PhD, and colleagues were the first to demonstrate the efficacy of intravitreal bevacizumab in patients with macular edema from CRVO.15 In their report, the authors treated a patient with macular edema from CRVO with an injection of 1 mg of bevacizumab. The visual acuity of the patient improved from 20/200 to 20/50 within one week, and the OCT showed a resolution of retinal edema. The treatment effect was maintained for four weeks.Since this initial report, several retrospective case series have demonstrated the short-term efficacy of the anti-VEGF agents for the treatment of macular edema associated with CRVO.16-18
Diana Iturralde, MD, and colleagues retrospectively evaluated the efficacy of bevacizumab for this indication in 16 consecutive eyes. They demonstrated success of this treatment in terms of decreasing macular edema and improving visual acuity.16After a mean follow-up of three months, the patients had received a mean of 2.8 injections. The mean central macular thickness (CMT) using OCT had decreased from 887 to 372 µm after the first month and the mean visual acuity improved from 20/600 to 20/138 by three months. No adverse events were noted during follow-up.16
Jason Hsu, MD, and colleagues retrospectively reviewed 30 eyes of 29 consecutive patients with macular edema associated with CRVO.17 Similar to the previous study, visual acuity improved throughout the length of follow-up (18 weeks). The authors claimed that the duration of the treatment effect of bevacizumab appeared to be about two months. No ocular or systemic adverse events were noted. Their data suggest that patients will require multiple injections with bevacizumab to maintain efficacy.
Ninel Z. Gregori, MD, and co-investigators at Bascom Palmer Eye Institute retrospectively reviewed the experience at that site with bevacizumab for macular edema from CRVO.18 They evaluated 57 eyes of 55 patients. The visual acuity improved by a mean of 14 letters at one month, 13 at three months, nine at six months, and nine at nine months. The mean CMT decreased by 299 µm at one month, 144 at three months, 127 at six months and 276 at nine months. By 12 months, each eye had received an average of 3.2 injections. No ocular or systemic adverse events were noted. The authors stated that compared with the visual acuity outcomes following treatment with intravitreal triamcinolone, the patients treated with bevacizumab seemed to experience better visual outcomes. Visual acuity improved by at least three lines in 41 percent of patients at one year.
Recently, results from a prospective study of the treatment of macular edema associated with CRVO with bevacizumab were published.19 Follow-up data at 12 months was available for six of eight eyes initially enrolled in this study. After one year, the mean best-corrected visual acuity had increased by seven letters, but this was not statistically significant. The central retinal thickness had decreased by 268 µm in the CRVO patients, however, and this was statistically significant. These study eyes received a mean of eight injections over the 12 months and re-treatment was indicated if the OCT showed evidence of intraretinal or subretinal fluid. No severe ocular or systemic complications were reported and no patient developed neovascularization of the disc, iris or elsewhere in the retina. The authors, however, recognized their small sample size and encouraged further studies using anti-VEGF agents prior to making generalized recommendations for the treatment of macular edema from CRVO.
• Steroid treatment. The recently published results from the SCORE trial demonstrated the efficacy of intravitreal triamcinolone to treat macular edema associated with CRVO. The study evaluated 271 patients with macular edema from CRVO and found that after one year of follow-up, intravitreal triamcinolone injections (1-mg dose) were more effective at improving visual acuity than observation.14 Twenty-seven percent of patients achieved a gain of 15 or more letters from baseline at month 12, using 1-mg injections of triamcinolone, while only 7 percent of those managed with observation experienced the same gain in visual acuity. While the average patient under observation lost 12.1 letters of vision, patients treated with 1 mg of triamcinolone lost an average of only 1.2 letters. The rates of cataract and elevation of the intraocular pressure were similar between the observation group and the 1-mg triamcinolone group.
The newest treatment option for macular edema from CRVO is an intravitreal 700-µg dexamethasone posterior segment drug delivery system called Ozurdex (Allergan). The implant was approved in June 2009 by the Food and Drug Administration to treat macular edema due to branch or central retinal vein occlusion. The most recent clinical trial data, from 12 months, was presented at February's Macula Society meeting.
In two identical, double-masked studies, patients with macular edema due to RVO (duration 1.5 to 9 months for CRVO, 1.5 to 12 months for BRVO) were randomized to receive the 700-µg dexamethosone implant, a 350-µg implant or sham. Patients could also receive open-label treatment with Ozurdex at six months if best-corrected visual acuity was <84 letters or retinal thickness by OCT was >250 µm. Patients entering the open-label phase (regardless of whether they were treated at six months) were followed for an additional six months. Key outcomes were BCVA, central retinal thickness, and safety
The 700-µg implant was found to be well-tolerated and produced substantial improvements in BCVA. The response to a second treatment after six months was similar to the response to the first treatment.
At baseline, 427 patients received the 700-µg implant and 426 received sham; at six months, 341/427 from the 700-µg group and 327/426 from the sham group entered the open-label phase and were treated with the 700-µg implant. Among patients who received two treatments with the 700-µg implant, 30 percent and 32 percent achieved ≥15-letter improvement 60 days (peak response) after the first and second injections, respectively. Among patients who received only a single treatment with the 700-µg implant at the beginning of the study and entered the open-label phase (n=80), the percentage who gained ≥15-letters was 28 percent at day 60, 45 percent at six months, and 39 percent at 12 months. Among patients who received their first treatment at six months, only 26 percent achieved ≥15-letter improvement 60 days posttreatment.
Increases in IOP were generally transient and similar following each treatment. Cataract adverse events occurred in 26 percent of patients treated with two injections of the 700-µg implant and in 5 percent of patients who received no treatment over the 12-month study.
Glaucoma
Due to the fact that glaucoma is a significant risk factor for the development of CRVO, many eyes with a new diagnosis of CRVO have existing glaucoma. These eyes require appropriate treatment of their glaucoma by a specialist.
Glaucoma may also arise as a complication of CRVO. Eyes with CRVO need to be closely monitored for signs of glaucoma because this disease may develop months after the vascular event.
In addition to measuring the intraocular pressure in these patients, gonioscopy should be performed initially to evaluate for neovascularization in the angle and the potential for angle closure. In eyes with a severe CRVO, there is a high incidence of iris neovascularization. This typically arises a mean of three to five months after the onset of symptoms.
The CVOS found that the most important risk factor that predisposed patients to iris neovascularization after CRVO was poor visual acuity.20 This study also found that panretinal photocoagulation, applied prior to the formation of any iris neovascularization, did not reduce the incidence of iris neovascularization in patients with an ischemic CRVO.20 In fact, about 20 percent of patients that received PRP prior to the development of iris neovascularization subsequently developed this complication despite the treatment.20 It was recommended by the CVOS study group to wait until iris, angle or retinal neovascularization developed before initiating PRP. The use of anti-VEGF agents may temper the neovascularization prior to the initiation of PRP. The CVOS study authors recommended that eyes with CRVO be monitored closely for six months for evidence of neovascular glaucoma.20 This can be achieved with serial undilated slit-lamp examinations and gonioscopy.
Surgical Management
Several surgical options exist to treat CRVO in the acute phase, however, the success of these treatments has been variable. The current surgical therapies consist of decompression of the central retinal vein by radial optic neurotomy and cannulation of the central retinal vein followed by infusion of tissue plasminogen activator. Radial optic neurotomy was developed with the intention of opening the scleral outlet to relieve pressure on the central retinal vein.21 The efficacy of these treatments has not been thoroughly demonstrated and therefore, these treatments are not routinely used.22
CRVO is a potential complication of carotid cavernous fistula with potentially devastating visual consequences. Risk factor modification and treatment of any predisposing systemic diseases continues to be of paramount importance. The treatment of its complications continues to evolve. For many years, the standard of care for the management of macular edema due to CRVO was observation. More recently, several alternatives have become available for the treatment of macular edema and neovascular glaucoma. These treatments have enabled the improvement of visual outcomes in many eyes following this devastating vascular event.
Dr. Witmer is a resident in the department of ophthalmology at the
1.
2. Kupersmith M. Techniques and surgical approach for transvenous embolization. Arch Ophthalmol 1996;114:750.
3. Kupersmith MJ, Berenstein A, Choi IS, et al. Management of nontraumatic vascular shunts involving the cavernous sinus. Ophthalmology 1988;95:121-30.
4. Goldberg RA, Goldey SH, Duckwiler G, Vinuela F. Management of cavernous sinus-dural fistulas. Indications and techniques for primary embolization via the superior ophthalmic vein. Arch Ophthalmol 1996;114:707-14.
5. Pollock S, Miller NR. Central retinal vein occlusion complicating spontaneous carotid-cavernous fistula. Arch Ophthalmol 1986;104(3):331.
6. Brunette I, Boghen D. Central retinal vein occlusion complicating spontaneous carotid-cavernous fistula. Case report. Arch Ophthalmol 1987;105:464-5.
7. Hines MW, Guy JR. Mechanism of visual loss in cavernous sinus thrombosis. Neurology 1986;36 (suppl 1): 249.
8. David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica 1988;197(2):69-74.
9. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in
10. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch Ophthalmol 2008;126:513-8.
11. Regillo C, Holekamp N, Johnson M, et al. Central Retinal Vein Occlusion, in American Academy of Ophthalmology, Basic and Clinical Science Course, Section 12, Retina and Vitreous.
12. Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol 1996;114(5):545-54.
13. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology 1995;102:1425-33.
14. Ip MS, Scott IU, VanVeldhuisen PC, et al. SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol 2009;127:1101-14.
15. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 2005;36:336-9.
16. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina 2006;26:279-84.
17. Hsu J, Kaiser RS, Sivalingam A, et al. Intravitreal bevacizumab (avastin) in central retinal vein occlusion. Retina 2007;27:1013-9.
18. Gregori NZ, Gaitan J, Rosenfeld PJ, et al. Long-term safety and efficacy of intravitreal bevacizumab (Avastin) for the management of central retinal vein occlusion. Retina 2008;28:1325-37.
19. Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol 2009;93(4):452-6. Epub 2008 Dec 15
20. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology 1995;102:1434-44.
21. Opremcak EM, Rehmar AJ, Ridenour CD, Kurz DE. Radial optic neurotomy for central retinal vein occlusion: 117 consecutive cases. Retina 2006;26:297-305.
22. Yamamoto T, Kamei M, Sakaguchi H, et al. Comparison of surgical treatments for central retinal vein occlusion; RON vs. cannulation of tissue plasminogen activator into the retinal vein. Retina 2009;29:1167-74.