Warning Signs
Surgeons say to watch out for these early and late signs of trouble. Any one of them might mean an anterior vitrectomy is in order.
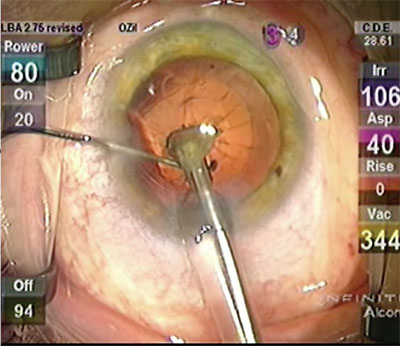 |
| A “spider” on the posterior capsule signifies that a break probably occurred and that an anterior vitrectomy is needed. |
“Sometimes it’s obvious the surgeon is going to have a problem,” says Minneapolis anterior segment surgeon Sherman Reeves. “They can directly see that they’ve created a tear in the posterior capsule, or they see the tear occur during irrigation/aspiration. Other times, however, it’s more challenging to identify, such as when the nucleus is being removed or during phaco in a very challenging cataract removal. Classic signs the surgeon can look for include a sudden deepening of the anterior chamber, which suggests that a posterior tear has occurred even though it might not be fully visible to the surgeon. Also, the iris may suddenly dilate, suggesting that a connection to the vitreous cavity has been created.
“Another sign is when the surgeon is removing lenticular fragments and he finds that the pieces aren’t coming to the phaco tip as they should,” Dr. Reeves adds. “The flow in the anterior segment is disturbed, abnormal. This suggests that vitreous is now in the anterior segment and is clogging the phaco probe. There could be an occult tear that the surgeon missed.”
Lisa Brothers Arbisser, MD, adjunct professor of ophthalmology at the University of Utah’s Moran Eye Center, lists a couple other late signs to be aware of:
• A nucleus that’s mobile but then, suddenly, isn’t mobile any longer, or develops a strange tilt.
• You touch one part of the tissue and another part moves. For instance, you touch the wound and see the iris move.
• The pupil is peaked or irregular.
• Your implant inexplicably won’t center.
• An incision is well-made but won’t seal. It could have a tiny strand of vitreous passing through it.
Surgeons say that your initial reactions to these warning signs can make a big difference in how successful the case turns out to be. “When you notice or even suspect the signs of a posterior capsular tear, the most important thing is to stop but not pull away—the moment you pull an irrigating instrument out of the chamber it will collapse, enlarging the capsular rent and encouraging vitreous to move forward,” advises Dr. Arbisser. “So, either stay in foot-position 1 or go to foot-position zero if the chamber is firm. Exchange the second instrument from the paracentesis for a visco cannula and fill the chamber with viscoelastic to maintain it. I prefer a dispersive viscoelastic but use whatever you have handy. Afterward, you can withdraw and inspect. The chamber should never be allowed to collapse again.”
Dr.
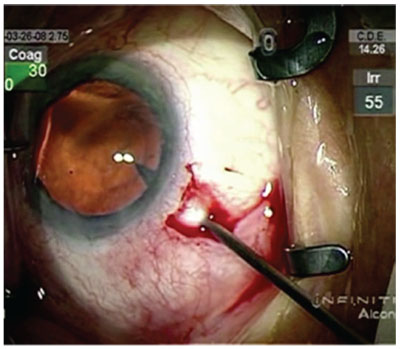 |
| For the entry stab incision, insert the blade until it’s visible in the pupil, surgeons say. |
Dr. Arbisser says that the surgeon should remember he or she has options to enhance visualization if a posterior tear occurs and vitreous comes forward. “This complication sometimes happens in the setting of a small pupil or capsulorhexis,” she says. “Don’t hesitate to retract the iris with a Kuglen hook in order to see what’s happening behind the iris. Visualization is everything. Iris hooks can also be placed, if necessary.
“The most important goal, and the point of every subsequent maneuver after recognizing this complication,” Dr. Arbisser continues, “is to prevent intraoperative or postoperative vitreous traction, which can lead to retinal tears and detachments, as well as cystoid macular edema. Also, keep a pressurized eye; hypotony or rapid, extreme pressure changes promote suprachoroidal hemorrhage, which can lead to permanently bad outcomes.”
Anterior Vitrectomy
When you’ve confirmed that a posterior tear has occurred, here are the experts’ tips on important aspects of your anterior vitrectomy:
• The incision. Some surgeons prefer an anterior approach while others like the advantages of a pars plana incision. “There are several good ways to approach it,” says Dr. Reeves. “The key pearl is, regardless of an anterior/limbal approach or a pars plana approach, the main cataract wound should be closed. That wound should be abandoned—at least temporarily—for the purpose of the anterior vitrectomy because it’s too large and would allow further vitreous prolapse through the wound. Therefore, it should be closed and probably have a suture put in it to further stabilize the anterior chamber.”
Alan Crandall, MD, director of glaucoma and cataract at the Moran Eye Center, notes the hazards of using the main wound. “Some surgeons will put a vitrector through the main wound,” he says. “But the wound is 2 to 3 mm, which is larger than the vitrector. This means there’s flow coming out of the wound that will encourage things to constantly be moving forward in the eye and tug on the retina. You always want a separate port so there’s a controlled anterior segment.”
Dr. Reeves says he usually uses an anterior approach. “I make a second limbal paracentesis—I’ve already got one from the cataract procedure—and do a
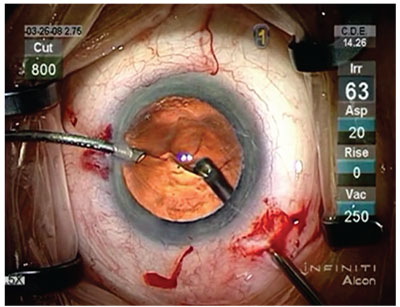 |
| For the vitrectomy, surgeons say to balance irrigation and linear vacuum with a flow rate of 20 cc/min. in foot-position 3. |
“Another key pearl is that in order to perform the most effective anterior vitrectomy you can, the coaxial vitrectomy should be set aside and a bimanual approach taken,” Dr. Reeves adds. “This applies whether it’s a limbal or a pars plana approach. Using two ports—one for irrigation and the other for the vitrector—gives the surgeon more freedom and access for vitreous removal, and also limits the size of the ports that are needed.”
Although she agrees with the need for a bimanual approach, Dr. Arbisser is quick to extol the virtues of the pars plana. “Regardless of the approach you use, the incision must fit the bare vitrector needle, as vitrectomy is done biaxially with the irrigation performed separately,” she notes. “It’s safest to always keep the irrigation in the anterior segment, because you want to keep the pressure higher anteriorly than posteriorly, since vitreous will always follow a gradient from high to low pressure. However, even if you try to put a vitrector through an anterior paracentesis down through the hole in the capsule, this has a tendency to not only call back vitreous but to call it forward as well and to enlarge the capsular rent. Therefore, a one-port pars plana approach is more efficient, stabilizes the posterior capsule rent, and increases the chance of amputating any anterior/posterior connections so that, if we need to sweep or Weck the wound, we don’t create traction.”
She feels that the use of a trocar may be even more helpful, since it allows the surgeon to operate transconjunctivally and sutureless, and provides a conduit that sticks into the globe without working right at the pars plana internal wall. This decreases the risk of incarcerating vitreous in the wound, and lets the surgeon work without any trauma or risk of detaching the choroid. “To insert the trocar, there are certain things to keep in mind,” Dr. Arbisser explains. “First, trocars require some pressure on the globe, so the globe has to be absolutely sealed. The trocar needs to be inserted parallel to, and 3.5 mm back from, the limbus. However, while a direct MVR blade incision is perpendicular to the limbus and sclera, with the trocar we’re going to be parallel to the limbus rather than perpendicular to it for a millimeter or two at a 30-degree angle to the sclera before going straight in, so the incision is 3.5 mm back at a 30-degree angle to the sclera and limbus-parallel. The goal is a scleral tunnel incision that has a roof and floor that will seal well and not allow vitreous to exit.”
• Vitrectomy. The surgeons say that it’s important to use the highest cutting rate possible when going after the vitreous strands to avoid traction on the retina. “I start by cleaning any vitreous in the anterior chamber that’s already prolapsed, using I/A mode,” explains Dr. Reeves. “Using the highest cut rate your machine can handle puts as little traction on the vitreous as possible as you try to clean out the anterior chamber. Then, I move to the posterior chamber and, at a very high cut rate, try to do a nice, complete core vitrectomy. Again, using the bimanual approach allows you to switch sides with the vitrector to make sure you’re getting the vitreous from all aspects of the anterior chamber and under the iris. Particular regions of interest are the subincisional areas, because those are where you’ll tend to have a lot of vitreous prolapse.” Surgeons say that, while in cut I/A, it’s often a good idea to remove vitreous in foot-position 3, but change to foot-position 2 when you’re not targeting vitreous but instead are just moving the vitrector, since this will avoid pulling vitreous with the instrument as it moves.
Surgeons say if the posterior tear is small, you may be able to create a posterior capsulorhexis and implant the IOL in the bag. “If there’s a small rent in the capsule, and you think you can manage implanting your lens in the bag, you can attempt to convert it to a posterior capsulorhexis using Utrata forceps,” says Dr. Crandall. “If you can do that, you can use a dispersive rather than cohesive viscoelastic because it will stay there while you complete your phaco.”
• Handling nucleus and cortex. Since the capsular tear can occur at various stages of cataract surgery, surgeons say you may have to deal with nuclear fragments and cortex during or after your vitrectomy. Here are their tips for leaving a clean anterior chamber. “If it’s a relatively small piece and I’m dealing with a capsular rupture, and the piece is still in the anterior chamber, I lower the bottle height significantly and use viscoelastic to bring the piece up,” says Dr. Crandall. “Then, even if I had to enlarge the wound to 3 or 4 mm, I’d just express it through the incision. That way I don’t have to risk going back in and hitting it with phaco. If it’s a larger piece, then I bury my phaco tip in it and bring it up. Then, using a second instrument, or something like a Sheets Glide or a Bobbit Glide to act as a pseudo-posterior capsule to keep it from falling back down, I can phaco it.” Dr. Crandall notes that sometimes nuclear fragments are hidden by the iris or are in the space between the iris and the anterior capsule. “As you start to clear vitreous, you may see these fragments come forward,” he says. “You have to constantly watch for them. If you see one, and it’s small enough, you can put visco in and bring it out. If it’s soft, though, you can switch the vitrector to I/A cut mode and treat it like epinuclear material for which you use just a little bit of flow. You can even do this with a phaco unit sometimes. However, in these instances, since you’ve got the vitrectomy handpiece going, as long as it doesn’t look like it’s going to drop, you can bring it up into the anterior chamber and deal with it there using I/A-cut mode.”
After the vitreous has been removed from the anterior chamber, surgeons say you can go after cortex. “After you’ve irrigated a small amount of Triessence [kenalog] into the anterior chamber and then blown it away with BSS irrigation, confirming the absence of prolapsed vitreous, you know you’ve reached your endpoint,” says Dr. Arbisser. “You can then place a nice layer of dispersive viscoelastic over the rent, and keep the bag open with a cohesive OVD. You now have three choices for cortex removal: The safest choice is to do it dry—i.e., use a 26-ga. cannula on a 3- or 5-mm syringe, target a little cortex and then strip it away in a cohesive-OVD-filled environment. This is safest because there’s no turbulence created. However, you have to keep filling the eye with OVD to keep it normotensive. Therefore, it’s not very efficient if you have lots of cortex.
“The next option is to
 |
| Lisa Arbisser, MD, says a wisp of vitreous will often be in the wound when an anterior approach is used for the vitrectomy. |
“The third, and most efficient, choice, is bimanual I/A,” Dr. Arbisser says. “With this, you attach a bimanual aspiration handpiece but keep the irrigation anterior. This keeps the pressure superior so as not to encourage more vitreous to come forward. You can then target the cortex, keeping in mind that you can’t allow the chamber to collapse, so you have to fill it with OVD before you come out. A meticulous cortex removal is valuable in these cases because they’re prone to inflammation, CME and fluffing up of the cortex, which can fall into the vitreous or obscure the visual axis.”
• Closing. Leaving a clean anterior chamber for any subsequent surgical intervention (such as by a retina colleague) is imperative, surgeons say.
“When you close the case, if you’ve left any nuclear material behind you want to place a stitch even if the wound appears stable and sealed,” Dr. Arbisser avers. “You want to set the stage for the retina surgeon to be able to go in and remove the nuclear pieces. Since he or she is going to be using a trocar, you want the wound stable. If the wound doesn’t seal easily, however, don’t just place a suture. It may have vitreous incarcerated in it, so you’ll need to instill Triessence to visualize any strands. Note that any sweeping or Wecking action will cause traction on the retina if vitreous is present. It’s best to go back into the vitrector incision (anterior paracentesis, temporary tied sclerotomy or plugged [valved] trocar) to amputate any anterior posterior connection or, if it’s a tiny strand, you can go in through another paracentesis, sharply cut the strand with scissors and push it back with some OVD.
“When removing viscoelastic after you’ve placed your IOL, it shouldn’t be done aggressively, as this can cause re-presentation of vitreous, unless optic capture is achieved, sealing the posterior segment,” Dr. Arbisser adds. “Miochol should be put in and a manual push/pull through the paracentesis performed. This is why I don’t use Healon V in complex situations, because you can’t leave it behind; but a little Viscoat left behind is more forgiving. In any case, it’s a good idea to do some hypertensive prophylaxis, so I’ll give a Diamox sequel if the patient isn’t allergic to sulfa drugs. I’ll also leave a little Triessence in the eye, placing it as a last maneuver to make sure no vitreous has come forward, as well as for its anti-inflammatory properties. Though it’s off-label, I believe we should use intracameral antibiotics. I recommend moxifloxacin 150 µg/0.1 ml as suggested by Steve Arshinoff, MD. Also, when the patient’s in the outpatient area, it’s a good idea to administer one oral dose of Avelox (moxifloxacin 400 mg)—except in cases where the patient has an allergy to it.” After surgery, she is aggressive with topical NSAIDs and a long steroid taper as an off-label cystoid macular edema prophylaxis. “A scleral indented exam is required, with timely referral to a vitreoretinal surgeon if there is residual nucleus,” Dr. Arbisser says.
Looking back over the process, Dr. Reeves says, “An anterior vitrectomy in an unplanned setting is always an anxiety-provoking moment.” However, he says that if you take your time, manage the anterior chamber well and carefully remove the vitreous, you can handle that moment successfully. REVIEW



