Daniel Dewey-Mattia and Kathy Crawford, PhD, Andover, Mass.
When you prescribe a drug, you're recommending a dosing schedule that was devised only after in-depth studies of the agent's pharmacokinetics, or the quantitative relationship between the biological effect of a drug and the observed plasma and tissue concentration. Even though the efficacy and safety of an ophthalmic medication is determined by its pharmacokinetic (PK) profile and there are numerous models and methodologies for researching the dynamic relationship between a drug and the various parts of the eye, PK remains something of a mystery to many. However, since ocular PK studies ultimately determine the standard amount of drug administered and the schedule of dosing, a knowledgeable clinician must understand how this information is obtained. Here's what you need to know about pharmacokinetics.
Animal Models of Ocular Pharmacokinetics
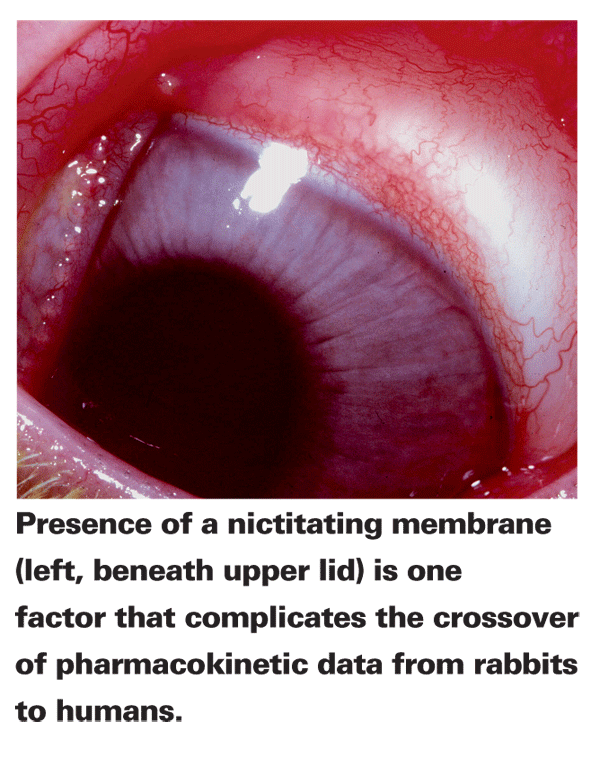
Unlike animal PK studies for systemic medications that are typically performed in mice or rats, ocular PK preclinical studies are usually done with rabbits, primarily because of their relatively large eyes. However, numerous physiological differences between rabbit and human eyes complicate the crossover of PK data. Rabbits only blink about four to five times an hour, while humans typically blink six to 15 times per minute. This slow blink rate can result in higher corneal surface drug concentrations and lower drainage.1 Rabbits are also less sensitive to alterations in solution viscosity and have a nictitating membrane not present in humans, which can absorb drug and act as a reservoir. Furthermore, albino species that lack eye pigmentation, a factor in PK, are most commonly used. These issues along with many other issues not mentioned here call into the question the viability of these PK studies. However, rabbits remain the animal of choice, as rodents are too small to test different delivery systems.
Topical Drug Delivery
Most ophthalmic drugs are administered topically. Eyedrops have major advantages, including increased localized drug effects and avoidance of the first-pass effect, in which the concentration of drug is reduced within the liver before reaching the target tissue. Topical administration, however, still results in low bioavailability to intraocular tissues due to physiological barriers that exist to protect the eye. Consequently, only 1 to 7 percent of a topically applied drug dose reaches the aqueous humor.2
Topical ophthalmic medication can be formulated in a suspension or in a solution. An instilled eyedrop is diluted by tear-film turnover and blinking, and most of an active ingredient will be transferred to the systemic circulation through the nasolacrimal duct within a few minutes.3 In the case of lipophillic drugs, about 50 to 80 percent of an instilled dose is absorbed into the systemic circulation.4
The cornea, conjunctiva and sclera serve as the primary routes of topical absorption. Hydrophilic drugs often reach target tissues via vessel uptake into the sclera and accumulation in the ciliary body. Lipophilic drugs, on the other hand, more easily penetrate across the cornea and diffuse through the pupil against aqueous flow to the posterior chamber.5
The Tear Film
Although the tear film is typically only about 7 µL in volume, a single eye drop is about 30 to 50 µL, depending on the surface tension characteristics of the solution. This excess liquid causes reflex blinking, spilling onto the cheeks and splashing to the eyelids, all of which decrease bioavailability.6 The tear film is also highly variable between individuals as there is up to a sixfold difference in tear flow. For example, dry-eye patients, who by definition have a deficiency in the quantity and/or quality of the tear film, may experience enhanced drug absorption due to the failure of this barrier function.
Research by my team has shown that the effect of a drug in normal subjects can be increased by administration after tear-film breakup. Pilocarpine instilled after tear-film breakup when the subject had refrained from blinking for six seconds resulted in a significantly greater decrease in pupil diameter over a 30-minute period compared to pilocarpine instilled before tear-film breakup. Likewise, administration of tropicamide after TFBUT caused significantly greater mean pupil dilation than tropicamide applied before TFBUT.7
Cornea and Active Transport
The cornea is usually the primary route of ocular penetration from the tear fluid to the anterior segment. In the case of lipophilic drugs, the ratio of corneal to noncorneal absorption is 70:1 for hydrocortisone, 12:1 for timolol and 5:1 for pilocarpine.3 However, the cornea also serves as a powerful barrier to drug absorption, especially for hydrophilic compounds, due to its relatively small surface area and low permeability.5
The hydrophobic membrane of the corneal epithelium is the single greatest obstacle to high bioavailability. While transporter-mediated drug delivery has historically taken a backseat to passive diffusion in the study of ocular pharmacokinetics, recent research has suggested that active transport may play a larger role in the passage of hydrophilic drugs because they are unable to passively diffuse through the epithelium.8 The importance of transporter-mediated absorption is debatable, however, since active diffusion is saturable and it's possible that once it's at full capacity, passive diffusion prevails.9
Two types of transporters that have been found to move drugs into and out of the cornea are multidrug resistance proteins (MRPs) and the protein-coupled oligopeptide transporter (POT) superfamily. MRPs are ATPase-powered transporters known to efflux organic anions and conjugated compounds, including ophthalmic medications.8 MRP1 and MRP3 may be the primary efflux barriers in the cornea, and designing drugs with enhanced resistance to their efflux may effectively enhance permeability.10 The POT superfamily, another group of active transporters, are influx transporters that have been used in prodrug technology.8 Using energy from the proton gradient, PEPT1 and PEPT2, two members of the POT superfamily, transport di- and tripeptides and various peptide-like drugs such as beta-lactam antibiotics, ACE-inhibitors, rennin inhibitors and viral nucleoside analogue prodrugs (such as valacyclovir and valgancyclovir).8
Unfortunately, corneal transporter-mediated uptake and elimination can differ greatly between species, making it more difficult to apply animal models of corneal absorption to humans.10 Relative expression of MRP1 efflux transporters is 10 times higher in the dog cornea than the human cornea.10 Likewise, relative PEPT1 influx transporter expression is more than 100 times higher in the human cornea than in the rabbit cornea. PEPT2 expression is 10 times higher.10 These wide disparities in transporter concentrations make it difficult (or even dangerous) to extrapolate pharmacokinetic values from work in animals.
Increasing Corneal Absorption
Various compounds can be added to topically administered ophthalmic drugs in order to enhance corneal absorption either by increasing corneal residue time or corneal penetration. Prodrugs with high corneal permeability can be chemically or enzymatically converted to an active drug after passing through the cornea. One classic example of a successful prodrug is dipivefrin, a lipophilic ester that, upon passage through the corneal epithelium, is hydrolyzed to produce epinephrine.11
The preservative benzalkonium chloride has also been suggested as a possible absorption enhancer, although evidence of a clinically relevant effect has only been found with exaggerated use. BAK has a high affinity for membrane proteins and can insert itself into the cellular membrane, possibly altering corneal ionic resistance. Researchers have speculated that this toxic effect at high doses can increase permeability, suggesting that higher BAK levels may cause better absorption and enhanced effect. However, a clinical study comparing BAK-free travaprost ophthalmic solution to travaprost with BAK found no significant difference in efficacy.12
Polymer penetration enhancers have also been used to increase drug absorption through the cornea. The cationic polymer compound chitosan hydrochloride was shown to significantly enhance intraocular penetration, possibly due to increased corneal permeability.13 Basic amino acids, such as L-arginine (PLA), appear to enhance the hydrophilic permeability of the cornea, conjunctiva and conjunctiva/sclera composite and thus may be useful in drug delivery via both corneal and noncorneal paths.14
Formulation Factors
Several factors in a drug's formulation can alter its ability to penetrate ocular tissues. Higher viscosity solutions have an increased dwell time on the ocular surface, allowing for longer absorption time. However, when the viscosity of a solution is increased over 70 cps, it can cause lid caking, blurring and discomfort.5 Attempts to increase bioavailability by increasing viscosity are almost always offset by increased drainage.5
Altering the lipophilicity and solubility of a compound can also enhance its ability to reach the target tissue. One example of this is moxifloxacin, which is not only highly lipophilic, but also highly soluble in water. Elevated lipophilicity compared to other fluoroquinolones allows moxifloxacin to pass more easily through the corneal tissues, while the enhanced aqueous solubility increases absorption by creating a strong concentration gradient between the tear film and corneal epithelium.15
Likewise, solution osmolality and pH can also affect absorption. Tears are slightly hypertonic at about 330 mOsm. Hypertonic solutions above 400 mOsm can produce discomfort and lacrimation, increasing loss of drug. Hypotonic solutions as low as 100 mOsm are still comfortable in the eye and may improve bioavailability of water-soluble drugs through the solvent-drag effect.5 Although converting an ionizable drug to a form at pH above or below the range of 6 to 8 may allow for more absorption, this will be offset by precorneal loss caused by discomfort and lacrimation.5 The comfort range of ocular solution pH is 6 to 8, and tissue damage occurs at pH below 3 or above 10.5
Alternative Forms of Delivery
Since standard eyedrop therapy is inefficient for the treatment of many ocular diseases, a number of new topical delivery systems are available or are being researched as alternatives. Here's a look at them.
New metered delivery systems, such as Visine Pure Tears, improve the design of the standard eyedropper by controlling drop size and allowing for the delivery of a multidose medication without the need for preservatives. Another novel metered delivery system in development is a small-volume nebulizer that delivers a mist to the eye.16 In a clinical study, the nebulizer system produced a significantly higher bioavailability of vitamin B12 than standard topical delivery.16
Another useful system is ocular inserts, which can be either biodegradable or non-biodegradable. Biodegradable inserts underneath the lid have been used to treat dry eye, but often show patient-to-patient variability in release kinetics due to different rates of tear production and turnover. Variability also arises from different concentrations of metabolic enzymes in the tear film and a final uncontrollable burst as the inserts degrade.9
Non-biodegradable inserts provide more precise control and longer periods of drug release than biodegradable systems. However, insoluble (non-biodegradable) inserts have failed to gain widespread acceptance. Ocusert Pilo, a pilocarpine-loaded, insoluble, non-surgical insert placed in either the upper or lower cul-de-sac for the treatment of glaucoma, for example, was costly and suffered from poor compliance due to ejection.5
An experimental method of drug delivery known as iontophoresis uses an electrical current to drive ionized drugs into cells. An iontophoresis device has been shown to be safe and well-tolerated in a clinical setting for the management of active corneal graft rejection, and it enhanced ocular absorption of small cationic compounds such as carboplatin.17
Two methods of ophthalmic delivery that incorporate an active ingredient in a transport vessel are liposomes and nanoparticles. Almost any type of drug can be encapsulated into liposomes, which are microscopic vesicles composed of one or more phospholipid bilayers. Liposomes bind to the cell membrane and facilitate drug transfer across it. They've been used to encapsulate the antiviral drug idoxuridine for treatment of herpes simplex keratitis.18 Nanoparticles are particulate polymeric drug delivery systems that are more stable than liposomes. Drugs can be incorporated into or absorbed by the particles, with smaller particles being more tolerable to patients. This mode of delivery can lead to higher drug absorption compared to ophthalmic solutions, due to its slower elimination rate.19
A basic understanding of pharmacokinetics is necessary when prescribing medications and considering dosing, as the PK profiles of ocular therapies play an important role in their safety and efficacy. New methods of delivery are being developed to overcome the poor bioavailability of topical solutions, and it seems likely that future therapies will bear little resemblance to the eyedrops of today.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surg Ophthalmol 1993;37:435-56.
2. Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv 2006;3:275-287
3. Shirasaki Y. Molecular design for enhancement of ocular penetration. J Pharmaceutical Sciences 2008;97:2462-96
4. Chang SC, Lee VHL. Nasal and conjunctival contributions to the systemic absorption of topical timolol in the pigmented rabbit: Implications in the design of strategies to maximize the ratio of ocular to systemic absorption. J Ocul Pharmacol 1987;3:159-69
5. Chun DK, Shapiro A,
6. Zaki I, Fitzgerald P, Hardy JG,
7. Abelson MB, Ousler GW. Instillation of ophthalmic agents after tear film break-up time to enhance treatment effect. Invest Ophthalmol Vis Sci 2001;42:4:S176.
8. Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood-retina barrier: Emerging role of transporters in ocular pharmacokinetics. Advanced Drug Delivery Reviews 2006;58:1136-63.
9. del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: A shift to the posterior segment. Drug Discovery Today 2008;13:135-143.
10. Zhang Y, Xiang CD, Gale D, et al. Drug transporter and cytochrome p450 mRNA expression in human ocular barriers: Implications for ocular disposition. Drug Metabolism and Disposition 2008;36:1300-7.
11. Mandell AI, Stentz F, Kitabchi AE. Dipivalyl epinephrine: A new pro-drug in the treatment of glaucoma 1978;85:268-75.
12. Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: A comparison of safety and efficacy. J Glaucoma 2007;16:98-103.
13. Di Colo G, Zambito Y, Burgalassi S, et al. Effect of chitosan and of N-carboxymethylchitosan on intraocular penetration of topically applied ofloxacin. Int J Pharm 2004;273:37-44.
14. Nemoto E, Takahashi H, Kobayashi D, et al. Effects of poly-L-arginine on the permeation of hydrophilic compounds through surface ocular tissues. Biol Pharm Bull 2006;29:155-60.
15. Robertson SM, Curtis MA, Schlech BA, et al. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv Ophthalmol 2005;50 Suppl 1:S32-45.
16. Kahn M. Bioavailability of vitamin B using a small-volume nebulizer ophthalmic drug delivery system. Clin Experiment Ophthalmol 2005;33:402-7.
17. Halhal M, Renard G, Coutois Y, et al. Iontophoresis: From the lab to the bed side. Exp Eye Res 2004;78:751-7.
18. Smolin G, Okumoto M, Feiler S, Condon D. Idoxuridine-liposome therapy for herpes simplex keratitis. Am J Ophthal 1981;91:220-5.
19. Diepold R, Kreuter J, Himber J, et al. Comparison of different models for the testing of pilocarpine eyedrops using conventional eyedrops and a novel depot formulation (nanoparticles). Graefes Arch Clin Exp Ophthalmol 1989;227:188-93.



