|
Baruch D. Kuppermann, MD, PhD |
Neovascular, or wet, age-related macular degeneration is responsible for visual disability in 80 percent of cases. Late-stage AMD causes an irreversible central visual loss, such as a disciform scar secondary to choroidal neovascularization and/or geographic atrophy. Current management options, such as laser photocoagulation and photodynamic therapy, are beneficial for preserving vision when compared with observation alone. Pharmacological inhibition of angiogenesis appears promising and may offer better outcomes.
All these treatments, however, are applicable in only a minority of cases and do not alter the natural course and prognosis of the disease itself. Thus, there is still central visual loss and this has a significant impact on daily activities such as reading or watching television, especially in patients with bilateral affection1 (45 to 55 percent of cases). Rehabilitation of these patients with the help of visual devices should be considered to improve the quality of their lives.
Visual Devices for AMD patients
Low vision aids are of two types: optical and non-optical systems.2 The optical systems are based on the principle of using magnification to increase image size on the retina. The currently available optical devices include high-plus lenses and external telescopes. A teledioptric lens system has also been investigated in the past.
 |
| Figure 1. The Implantable Miniature Telescope, developed by Isaac Lipshitz, MD. |
A recently developed miniaturized implantable telescope may outweigh some limitations of existing technology and improve central vision in these patients.
Implantable Miniature Telescope
The Implantable Miniature Telescope (IMT by Isaac Lipshitz, MD) (See Figure 1) is a visual prosthetic device designed for placement in the capsular bag after cataract extraction.4 The research, development, manufacturing and marketing of the telescope is being done by VisionCare Ophthalmic Technologies Inc., a private, venture capital-funded company headquartered in Saratoga, Calif. The innovative device, devised by company founders Dr. Lipshitz and Yossi Gross, consists of a micro-sized telescope, 4.4-mm long and 3.6 mm in diameter, contained in a polymethylmethacrylate carrier with two continuous haptics, which facilitate in-the-bag fixation. The telescope is made of an anteriorly positioned positive micro lens, posteriorly positioned negative micro lens, and a "bubble of air" in between sealed in a glass cylinder with clear window end caps. The air bubble helps to float the device in aqueous, thereby decreasing its weight from approximately 120 mg in air to 60 mg in the aqueous.
The device's optics are configured for a standard human eye and, in conjunction with the cornea, it is designed to optimally focus at approximately 3 m from the patient's eye, set for intermediate distance activities. It functions as a telephoto system and provides 3X or 2.2X retinal image enlargement of the central visual field. This can be further enhanced to patient requirements by standard external glasses, such as for reading or other near activities. The optics of this device are such that a high-resolution enlarged image is projected onto approximately 56 degrees of the central and peripheral retina, the depth of field is large, and there is minimal image distortion with a 22-degree field of view.
Indications, Patient Characteristics
The implantable telescope is designed for monocular use in patients with bilateral central visual field loss secondary to stable late-stage AMD. The implanted eye provides central vision while the fellow eye provides peripheral vision. The best-corrected visual acuity should be between 20/80 and 20/800 in both eyes, and the eye under consideration for the implant should have an improvement in visual acuity with an external telescope.
Moreover, patients must have evidence of cataract, sound mental health and willingness to participate in vision training to adapt to their new visual status during the first few postoperative months. Patients excluded from current studies include those with: significant corneal disorders (endothelial count < 1,600 cells/ mm2); high refractive errors (> 6 D); past intraocular and/or refractive surgery; uncontrolled glaucoma; zonular weakness; or other significant retinovascular or vitreoretinal disorder.
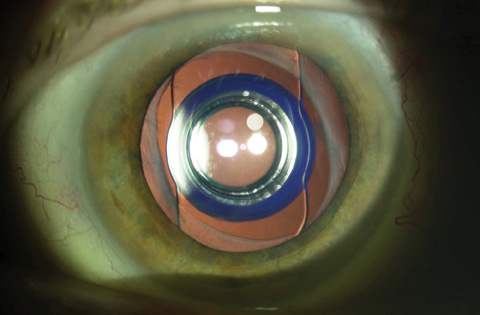 |
| Figure 2. Dilated anterior segment photograph of the IMT-implanted eye showing a fibrosed capsular bag around the carrier plate. |
Preop Workup, Surgical Technique
A thorough ophthalmic evaluation is essential including visual acuity (distance and near), slit lamp examination, cataract evaluation, fundus photography, fluorescein angiography, intraocular pressure measurement, A-scan, corneal pachymetry, specular microscopy and corneal topography. Surgical implantation is performed by a skillful anterior segment surgeon, either by a planned extracapsular cataract extraction through a limbal incision or phacoemulsification through a scleral tunnel.4 In both the techniques, the incision length should be at least 10 to 11 mm, and there should be a large continuous curvilinear capsulorhexis of at least 6.5 mm to accommodate the length of the telescope.
During insertion of the device, the anterior chamber should be generously filled with cohesive viscoelastics to prevent corneal endothelial touch and promote easy insertion. Handling of the central glass optic of the device should be avoided and the haptics should be placed at 12:6 o'clock position after insertion in the capsular bag to ensure stability. A peripheral iridectomy should always be performed to prevent potential pupillary block glaucoma in the postoperative period.
At the end of the surgery, a properly positioned implantable telescope marginally protrudes through the pupil by approximately 0.1 to 0.5 mm allowing for a clearance of approximately 2 to 3 mm from the posterior cornea, avoiding damage to the endothelium. Operative times are substantially longer than common anterior segment surgery. Generous use of cohesive viscoelastics is advised and routine intraoperative and postoperative medications (topical antibiotic and steroid) are used. Due to the large incision, however, steroids are used longer and then tapered at two months in the absence of any significant inflammation.
Clinical Trials
A Phase I multicenter clinical trial of the implantable telescope was conducted at four centers in the United States to evaluate the safety and effectiveness of the predecessor non-wide angle version of the device.6 The optical portion of this earlier version was slightly longer and narrower (4.6-mm long, 3-mm diameter) with a smaller field of view (12 degrees).
A total of 14 patients (³60 years of age) with stable, bilateral disciform scar or atrophic AMD, cataract, and visual acuity between 20/80 and 20/400 were implanted with the IMT. Distance and near BCVA, endothelial cell density (ECD), and activities of daily living (ADL) were assessed at baseline, three, six and 12 months. The mean patient age was 80 and all the patients were Caucasian; 64 percent were female. All patients completed all follow-up visits except for one patient who died prior to the 12-month visit. At six months, seven of 13 (54 percent) and six of 13 (46 percent) experienced a gain of two lines or more of distance or near BCVA, respectively.
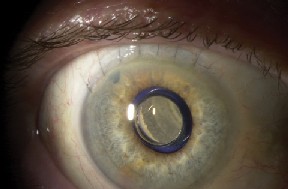 |
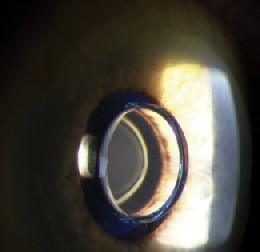 |
| Figures 3a and 3b. Postoperative photograph after implantation by limbal approach. The IMT is properly positioned and the anterior micro lens is seen behind the front window of the optical cylinder. | |
At 12 months, 10 of 13 patients (77 percent) experienced a gain of two lines or more in either distance or near BCVA and eight of 13 (62 percent) gained three or more lines in either distance or near BCVA. Mean ECD decreased from baseline by 7 percent at three months, 13 percent at six months, with no further cell loss reported at 12 months.
There were several reports of persisting intraocular inflammation, which resolved with appropriate steroid treatment without any permanent sequelae, except for a single eye with persistent anterior uveitis that resolved after 12 months. ADL improved by 29 percent and 55 percent in those patients who experienced an improvement in either distance or near BCVA of ³ 2 or ³ 3 lines, respectively, at 12 months.
These initial promising results with the implantable telescope prompted a Phase II/III trial evaluating two wide-angle models that provide 2.2X and 3X retinal image enlargement and are designed to have double the field of view of the device tested in Phase I. The trial has completed patient enrollment of more than 200 subjects with bilateral late-stage AMD with distance BCVA no better than 20/80 and no worse than 20/800 in either eye at 28 investigational sites in the United States.
The primary efficacy outcome measure is ³ 2-line improvement in either near or distance BCVA (measured by ETDRS) in 50 percent of implanted eyes 12 months post-implantation. A secondary outcome measure is improvement in quality of life (ADL and NEI VFQ-25). The primary safety outcomes are mean endothelial cell loss not to exceed 17 percent, and loss of > 2 lines in near or distance BCVA in £ 10 percent of implanted eyes without corresponding improvement in either near or distance BCVA. Early results from this phase II/III trial are expected in the middle of 2004.
Merits and Demerits of the IMT
The implantable telescope is a visual prosthetic device unlike external devices. It is a permanent solution that involves no relative movement between the eye and the telescope, eliminating the problem of image blurring from eye movements. It also eliminates the optical aberrations and distortion, as there is minimal chance of decentration or tilt after proper in-the-bag placement of the prosthetic device. Moreover, it obviates other disadvantages of external devices, such as difficult handling, weight and poor cosmetic appearance.
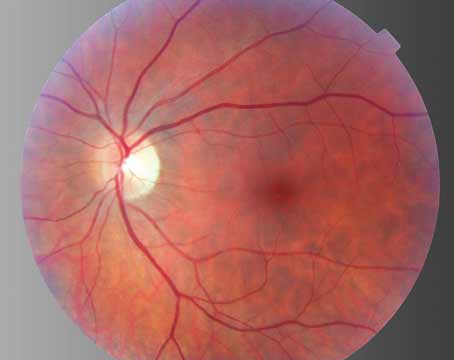
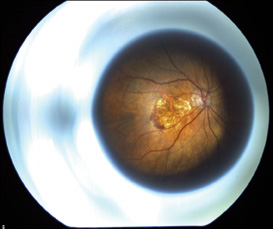
The optics of the device is such that it is possible to obtain fundus photographs (See Figure 4a) and fluorescein angiogram (See Figure 4b) through the telescope to detect progression of AMD or other posterior segment pathology. Despite the ability to take fundus photos through the IMT, visualizing the macula through the IMT optics can be challenging. Laser treatment of the retina and retinal surgery in the presence of the IMT may be challenging as well. No cases of new CNV or retinal detachment occurred in the Phase I trial. Outcomes from the larger Phase II/III trial will give vitreoretinal specialists a better perspective on managing IMT-implanted eyes.
 |
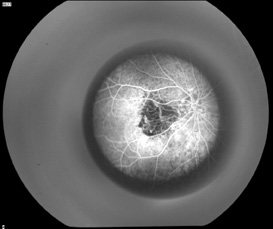 |
| Figures 4a and 4b. Fundus photograph of left eye through the IMT showing a disciform scar and the early phase fluorescein angiogram through the IMT showing an area of blocked fluorescence corresponding to the disciform scar. | |
In spite of all these advantages, some patients may not be satisfied with the quality of their postoperative vision due to expectations of a "cure" or potential disparity between the near and distance acuities. This could be due to the scotoma size, with larger scotomas increasing the chance of a continued, yet smaller, scotoma and the acuity disparity. Psychosocial status of the patient is also an important consideration, one that influences the efficacy outcomes in the postoperative period. Thus, proper selection of patients with realistic expectations and a willingness to participate in vision training and counseling are essential.
The implantable telescope is a novel device and has a positive impact on the visual acuity and quality of life in patients with late-stage AMD. A multidisciplinary approach involving a retina specialist, an anterior segment surgeon and a low-vision specialist is essential for the care of these patients. A realistic explanation of the goals and obstacles to the patients is necessary to maximize functional benefits.
The results of Phase II/III trials will unlock the door to more targeted and effective management of patients with late-stage AMD.
The authors would like to thank Daniel Berinstein, MD, for providing the fundus photograph and fluorescein angiogram taken through IMT. Drs. Kuppermann and Kulkarni are in the Department of Ophthalmology at the University of California, Irvine. Dr. Fine is in the Department of Ophthalmology at the Oregon Health & Science University. Contact Dr. Kuppermann at 118 Med Surge I, Irvine, CA 92697. Phone: 949-824-6256; fax: 949-824-4015; e-mail: bdkupper@uci.edu.
1. Wang JJ, Mitchell P, Smith W, Cumming RG. Bilateral involvement by age related maculopathy lesions in a population. Br J Ophthalmol 1998;82:743-7.
2. Peyman GA, Koziol J. Age-related macular degeneration and its management. J Cataract Refract Surg 1988;14(4):421-30.
3. Koziol JE, Peyman GA, Cionni R, et al. Evaluation and implantation of a teledioptric lens system for cataract and age-related macular degeneration. Ophthalmic Surg 1994; 25:675-84.
4. Lipshitz I, Lowenstein A, Reingewirtz M, Lazar M. An intraocular telescopic lens for macular degeneration. Ophthalmic Surg Lasers 1997; 28:513-17.
5. Kaskaloglu M, Uretmen O, Yagci A. Medium-term results of implantable miniaturized telescopes in eyes with age-related macular degeneration. J Cataract Refract Surg 2001;27(11):1751-5.
6. Lane SS, Kuppermann BD, Fine IH, Hamill MB, Gordon JF, Chuck RS, Hoffman RS, Packer M, Koch DD. A Prospective Multicenter Clinical Trial to Evaluate the Safety and Effectiveness of the Implantable Miniature Telescope (IMT). Am J Ophthalmol – in press.