In the 1930s, Frederic Mohs, MD, brought about a revolution in cancer surgery by showing how surgeons could remove an entire tumor while leaving the maximum amount of healthy tissue behind. This led to excellent cure rates and minimized disfigurement for the patient. Similarly, cornea specialists are hard at work perfecting their own brand of selective surgeries to replace full-thickness grafts. These advanced techniques strive to remove just the diseased or scarred tissue while leaving healthy native cornea behind.
Here are the latest techniques in Descemet's stripping automated endothelial keratoplasty and deep anterior lamellar keratoplasty that surgeons are using to enhance outcomes and minimize graft failures.
Replacing the Endothelium
In the spirit of only removing what you need to for endothelial transplantation, transplant surgery has evolved into the current state-of-the-art: DSAEK. In DSAEK, the recipient's Descemet's membrane and endothelial cells are stripped away and replaced by healthy tissue from a donor.
Portland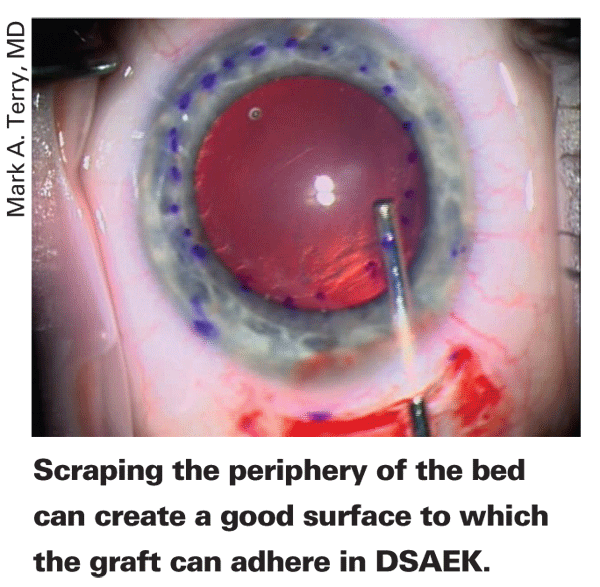
In the DSAEK procedure developed by Dr. Terry, the surgeon makes a 5-mm-long scleral incision located 1 mm or less posterior to the limbus on the temporal side of the recipient eye. He then uses a crescent blade to create a tunnel into clear cornea along the incision's length. He makes an 8- to 9-mm diameter circular template mark on the epithelium. Through a paracentesis, the surgeon injects Healon into the anterior chamber, and uses a blunt reverse Terry-Sinksey hook (Bausch and Lomb) to score peripheral Descemet's for 360 degrees, just central to the template ring. The same hook is then used to strip Descemet's from each quadrant toward the center. The blunt hook helps ensure that the overlying stroma isn't engaged and makes scoring and stripping easier. He uses forceps to remove Descemet's and he also creates another paracentesis on the other side of the main incision for use later.
He introduces a Terry Scraper (Bausch & Lomb) through the wound and uses it to roughen the peripheral 1 mm of the recipient bed. This step is aimed at promoting adherence of the graft by giving it a roughened peripheral edge to stick to. The surgeon then widens the scleral incision entrance into the anterior chamber to 5 mm. He then aspirates out the Healon with an irrigation/aspiration device and injects Miochol to constrict the pupil. The smaller pupil helps facilitate safe donor insertion later on as well as promoting a more stable air bubble during final positioning and adherence of the graft.
After the donor cap is cut with a microkeratome, the cap is placed back into position and the tissue is placed endothelial side up onto a donor punch block. A same-size button (8 or 8.5 or 9.0 mm) is punched out with a trephine; the surgeon puts a light line of Healon horizontally across the endothelium of the graft, and then folds it in a 60/40, taco configuration. Inserting it with the 60-percent side anteriorly avoids accidental upside-down unfolding of the graft later.
The surgeon inserts the graft into the chamber with the stromal side of the taco on top and the taco's opening facing to the left. He then unfolds the graft using balanced salt solution and air. He injects the BSS through a paracentesis to deepen the chamber and unfold the graft, stopping the BSS just prior to the graft reaching a 90-degree configuration with the 40-percent stromal side nearly perpendicular to the iris. At this point, he injects air through the other paracentesis into the taco, causing it to open and elevating it into position.
He then uses limbus-to-limbus surface compression sweeping with the Cindy Sweeper (Bausch & Lomb) to center the graft. When it's centered, the surgeon secures the wound with interrupted sutures and fills the chamber completely with air to an elevated pressure to secure the tissue. Now, he tries to remove any fluid that remains in the interface using the Cindy Sweeper. He gently strokes the surface, compressing the corneal surface from center to periphery in each quadrant. He places dilating drops at this time to help avoid pupillary block in the postoperative period, and then waits for 10 minutes with the microscope light off to allow the graft to settle into place and enhance attachment. At the end of that period, he removes the large air bubble completely, taking care to remove all of the air from behind the iris and from the anterior chamber. Dr. Terry points out that the tissue "should stay in place with absolutely no air support at this point." After normalizing the pressure with BSS he then injects enough air to create a 6.5 to 7 mm, fully mobile bubble. He closes the conjunctiva with cautery and places a collagen shield soaked in antibiotics. The patient lies supine for an hour postop and then is allowed to get up and go home.
Though Dr. Terry says it's hard to isolate the key steps that allow his procedure to work, he says that he'd probably say two of the most important are the 5-mm incision and the peripheral scraping. "We have a paper that's accepted for publication in which we find that incisions smaller than 5 mm cause too much damage to the endothelium," he says. Finally, Dr. Terry emphasizes the importance of removing all the air from the eye at the close and reinjecting just enough for a freely mobile air bubble. "Because it's mobile, it doesn't attach to the pupillary margin," he says, "so it can't cover the pupil when the patient sits up and induce pupillary block."
Other surgeons have added their own tweaks to the basic procedure. Mark Gorovoy, MD, of
In addition to matters of technique, there's currently a controversy among DSAEK surgeons about whether it's better to get the donor tissue precut at the eye bank or cut it themselves.
"I prefer to cut my own because then I control the whole process," says Dr. Gorovoy. "That way, I know how large the anterior cut is. And it's going to be fresh, with little storage media getting into the stromal interface."
Dr. Price also prefers to cut his own. "I don't think there's anything wrong with precut tissue, I just prefer doing my own, he says. We have the equipment and we like to be in control. For instance, if you get a small-diameter cut, and you see it yourself, you can make the diameter larger with a hand lamellar dissection that extends the depth of the bed out into the periphery of the donor cornea while the tissue is still on the artificial anterior chamber. The downside is you can't recoup the $3,000 cost if you mess up a cut, which is an obvious disadvantage for surgeons who aren't familiar with using a microkeratome. Also, the equipment is kind of expensive, so if you don't do many in a year, it may be hard for you to justify cutting the tissue yourself."
Dr. Terry, however, thinks precut tissue from the eye bank has some significant advantages. "If a surgeon cuts tissue in the operating room with a microkeratome, he has no ability to examine the tissue at the slit lamp or with a specular microscope to determine the actual health of the tissue after the cut," he avers. "There may be endothelial damage from the microkeratome cut that he'd be oblivious to. When the donor is cut at the eye bank, though, it's put back in storage media, and can be examined by slit lamp or specular microscopy, because it's not going to be used until the next day." Dr. Terry says recent data from his group have shown precut tissue has the same low, 1-percent dislocation rate he's reported before, as well as outcomes that are identical to those of surgeon-prepared tissue.3
The idea of surgeons precutting their own tissue may be a moot point, however, come January 2009.
"After the first of the year, the Centers for Medicare & Medicaid Services are going to issue new codes that could financially penalize surgeons who cut their own, pushing everyone to use precut tissue," Dr. Price. "We don't know for sure, but we've been told we won't get paid as much to precut the tissue as the eye banks will get."
The other area of evolution in DSAEK is the use of instruments other than forceps to implant the donor graft. These primarily include the Busin and Sheets glides and injectors.
"The Sheets glide is a strip of plastic that's been used for years to guide intraocular lenses into the eye," says Dr. Price. "There are some advantages to using it in DSAEK, such as the donor always goes in right side up and it's easier to get it to unfold than with a pair of forceps. The glide goes across the incision to the far side of the anterior chamber. You have to use forceps to pull in the graft from the far side of the chamber. It appears to be less traumatic than forceps for the insertion."
As far as injectors go, none of them are currently available for sale, but instead are in various stages of development (See August 2008's Technology Update for a detailed description of them). Also, none of them currently have endothelial cell count data. The developers say this data will be forthcoming.
The Advent of DMEK
The next evolution of endothelial transplantation may come in the form of a procedure developed by the
Dr. Melles describes the difference between DMEK and DSAEK: "In DMEK, we also start with making a descemetorhexis, " he says. "Then, instead of implanting a taco- or burrito-shaped posterior disk consisting of posterior stroma, Descemet's membrane and endothelium, we inject a donor Descemet's with its endothelium. After stripping the donor Descemet's membrane from a corneo-scleral button, it rolls up by itself, so the implantation can be performed through a 3-mm limbal incision."
The main advantage of DMEK, according to surgeons familiar with it, is that when successful, vision may be better than it is post-DSAEK, since no stroma is transplanted. Dr. Melles says that, in his experience, one to three months post-DMEK, 90 percent of cases that have good visual potential preop see 20/40 or better and 60 percent see 20/25 or better. However, the surgery is very new, and surgeons are waiting for more data.
Dr. Price has done several DMEKs. "I think DMEK is more labor-intensive than DSAEK at this point," he says. "The tissue is very thin and difficult to handle. That's probably the biggest problem; it's easy to damage the endothelium or put it into shock, and Descemet's is rather brittle. We've been working with techniques developed by Arthur Giebel, MD, of
Everything But the Endothelium
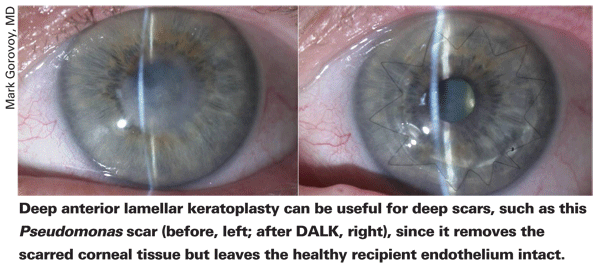
The other evolution in corneal transplantation, deep anterior lamellar keratoplasty, hasn't seen as many tweaks that enable surgeons to enjoy the more consistent success that they do with DSAEK. For patients with deep anterior corneal pathology, such as deep scars, keratoconus and atopy, DALK involves dissecting down to Descemet's but no farther, which removes all the anterior layers but retains the recipient eye's natural, undamaged endothelium. An anterior graft, its own Descemet's membrane stripped, is then sutured in place atop the recipient's Descemet's. This is the main benefit of a successful DALK; by retaining the endothelium, there is no chance for graft rejection, as might be the case if one were to do a penetrating keratoplasty. However, the benefit of DALK is also its toughest part, since it's difficult to accurately dissect down to Descemet's without puncturing it. The main advances in DALK have been the Anwar big-bubble technique and the use of femtosecond lasers for donor and host preparation. (The last advance is covered in detail in this issue's feature, "Laser-Enabled Keratoplasty: Shaping the Future?" )
"We're still searching for the best way to reliably remove tissue and leave behind intact Descemet's membrane," says Duke corneal specialist Alan Carlson. "That can be a fairly tedious process."
The big-bubble technique was developed by Mohammed Anwar, DO, FRCS, of
"The big bubble technique has been a huge boon for keratoconus and scar treatment," says Dr. Price. "However, it doesn't always work. The surgeon has to be comfortable with doing a hand dissection right down next to Descemet's in order to avoid having to convert to a PK. Having a slit attachment on the operating microscope can help, because it gives you a reference point for depth judgment. Also, Gerrit Melles came up with the idea of putting some air into the anterior chamber to provide a reflection between the bubble and the underlying air. A few smaller bubbles in the chamber can help show you the extent of the big bubble or how deep you are if you're dissecting by hand."
Dr. Terry says he's developed some tips as well. "Once I get the big bubble, I inject Healon into that space so I can keep Descemet's attached when I remove the recipient cornea," he says. "You also have to keep the eye exceedingly soft during the time you're taking off the recipient tissue by milking the paracentesis site of aqueous."
Though the new transplant techniques can be technically demanding, surgeons feel selective keratoplasty is the way of the future. "When you think about the cornea, it only has four roles," says Dr. Carlson. "It maintains structural integrity, transmits light, refracts light and provides a pleasing cosmetic appearance that allows others to see a normal ocular anatomy. So, when you have a cornea that's going bad, if you can re-establish these roles with the least amount of added tissue, you've done a good job."
1. Terry MA, Shamie N, Chen ES, Hoar KL, Friend DJ. Endothelial keratoplasty: A simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology 2008;115:7:1179-86.
2. Kitzmann AS, Goins KM, Reed C, Padnick-Silver L, Macsai M, Sutphin JE. Eye Bank Survey of Surgeons Using Precut Donor Tissue for Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2008;27:6:634-639.
3. Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Precut tissue in Descemet's stripping automated endothelial keratoplasty donor characteristics and early postoperative complications. Ophthalmology 2008;115:3:497-502.
4. Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg 2002;28:3:398.