Intravitreal pegaptanib sodium (Macugen), an RNA aptamer designed to target VEGF 165 and larger isoforms, and intravitreal ranibizumab (Lucentis), a monoclonal antibody antigen-binding fragment that targets all isoforms of VEGF and their smaller bioactive cleavage products, received Food and Drug Administration approval for the treatment of neovascular age-related macular degeneration in 2004 and 2006, respectively. Bevacizumab (Avastin), a full-length humanized monoclonal antibody that targets all isoforms and active cleavage products of VEGF-A, is FDA-approved in combination with chemotherapy for the systemic treatment of metastatic colorectal cancer and lung cancer. Intravitreal bevacizumab is used off-label for the treatment of neovascular AMD.
The successful treatment of neovascular AMD with these three anti-VEGF agents—pegaptanib sodium, ranibizumab, and bevacizumab—has ushered in a new paradigm for AMD treatment, in which frequent intraocular injection of these effective anti-VEGF therapies has become commonplace. However, the ophthalmic community desperately needs new treatment regimens that alleviate the burden of frequent intravitreal injection, either through longer-lasting inhibition of VEGF or through combinations of regimens that inhibit other important factors in choroidal neovascular membrane formation and growth.
VEGF-A (also referred to in the literature as VEGF) is certainly a major factor in the angiogenic process and represents the target of much ongoing research. VEGF-A is potent regulator of both angiogenesis and vascular permeability. It is one member of the VEGF family of growth factors that also includes VEGF-B, VEGF-C, VEGF-D and placenta growth factor (PlGF). Alternative RNA splicing of the human VEGF-A gene results in the formation of four major isoforms—VEGF 121, VEGF 165, VEGF 189 and VEGF 206—and at least five minor isoforms—VEGF 145, VEGF 148, VEGF 162, VEGF 165b and VEGF 183. VEGF 165 and larger isoforms contain heparin-binding domains that tether them to the extracellular matrix, although about 30 percent to 50 percent of VEGF165 is diffusible. VEGF-A isoforms smaller than VEGF 165, such as VEGF 121, are diffusible. Plasmin and metalloproteinase cleavage of the larger VEGF-A isoforms produces diffusible bioactive cleavage products, VEGF 110 and VEGF 113, respectively. VEGF 165 and VEGF 121 have been suggested to have the strongest mitogenic and vascular permeability-promoting potential.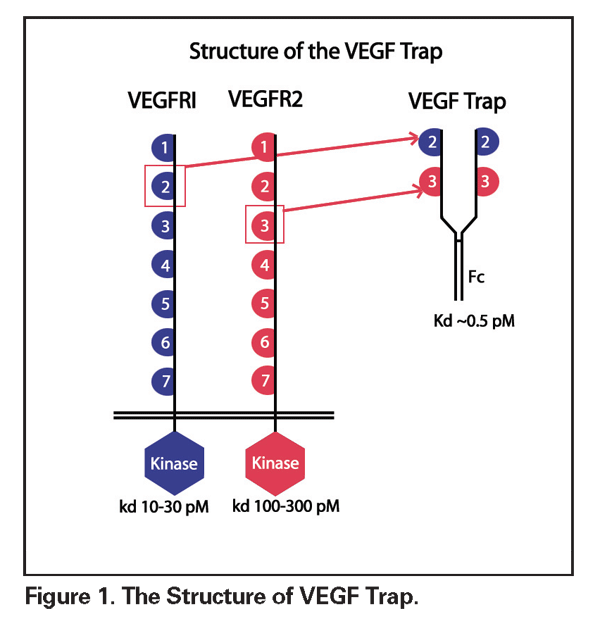
A substantial amount of evidence from preclinical and clinical studies implicates VEGF-A in the pathogenesis of neovascular ocular diseases. In preclinical studies, VEGF-A gene expression was shown to be significantly increased in the ganglion and inner nuclear retinal cell layers in streptozotocin-induced diabetic rats compared with control rats. Laser-induced retinal vein occlusion in rabbits and monkeys also led to increased expression of VEGF-A mRNA, and in the rabbit model, VEGF-A expression was specifically localized to ischemic regions of the retinal layers affected by laser treatment. Furthermore, inhibition of VEGF-A prevented retinal neovascularization in an ischemia-induced mouse model. In clinical studies, increased expression of VEGF-A was found: in the retinal pigment epithelium, subfoveal fibroblasts and surgically excised CNV membranes of eyes from patients with neovascular AMD; in the aqueous and vitreous fluid of patients with diabetic retinopathy, CRVO, BRVO, iris neovascularization, retinal detachment and ROP; and in all nuclear layers of the retina of ischemic CRVO eyes.
VEGF Trap Enters Phase III
VEGF Trap (Regeneron Pharmaceuticals) is a high-affinity recombinant fusion protein that consists of the immunoglobulin domain 2 of the VEGF-R1 receptor and the domain 3 of the VEGF-R2 receptor, fused to the crystallizable fragment of human IgG (See Figure 1). This antigen selectively binds and neutralizes all exogenous VEGF-A molecular isoforms as well as placental growth factor. VEGF Trap functions as a potent decoy receptor, binding VEGF more tightly than either native receptors or monoclonal antibodies. Administration can be either local or intravenous. In preclinical evaluations, VEGF Trap was evaluated as a possible antiangiogenic agent in tumor therapy. Using murine choroidal and retinal neovascularization models, Peter Campochiaro, MD, and associates determined that this agent inhibited CNV formation, pre-retinal neovascularization and retinal vascular leakage, as well as reducing blood retinal barrier breakdown.
Recently, Regeneron announced positive primary endpoint results from a Phase II study of VEGF Trap in neovascular AMD. This double-masked, prospective, randomized, multicenter trial enrolled 157 patients in five groups, with two groups receiving monthly doses of 0.5 or 2 mg, and three groups receiving quarterly doses of 0.5, 2 or 4 mg. Patients were monitored for safety, retinal thickness, and visual acuity. All five dose groups showed an improvement in retinal thickness and an increase in mean letters read versus baseline at all time points through week 12. There were no drug-related ocular or systemic serious adverse events reported. The study met the primary endpoint of a statistically significant reduction in retinal thickness after 12 weeks of treatment compared with baseline (all five dose groups combined, mean decrease of 119 µm, p<0.0001). The mean change from baseline in visual acuity, a key secondary endpoint of the study, also demonstrated statistically significant improvement (all groups combined, increase of 5.7 letters, p<0.0001). VEGF Trap may have a long duration of action, as the single 2-mg dose maintained a similar effect on visual acuity as 2 mg dosed monthly out to eight weeks (5.8 vs. 6.2 letters gained at eight weeks, respectively).
With these promising results, a Phase III trial has recently begun, comparing VEGF Trap to ranibizumab. In this clinical trial, VEGF Trap will be assessed using four- and eight-week dosing intervals in direct comparison with ranibizumab administered every four weeks according to its label. The trial will involve 1,200 subjects randomized to three VEGF Trap groups (0.5 mg every four weeks weeks, 2 mg every four weeks, 2 mg every eight weeks) and one Lucentis group (0.5 mg every four weeks). The subjects will be followed monthly to week 52 with assessment of visual acuity, optical coherence tomography and angiography. The study is powered to demonstrate statistically significant non-inferiority to Lucentis (0.5 mg every four weeks). The trial will involve 193 sites within and outside the 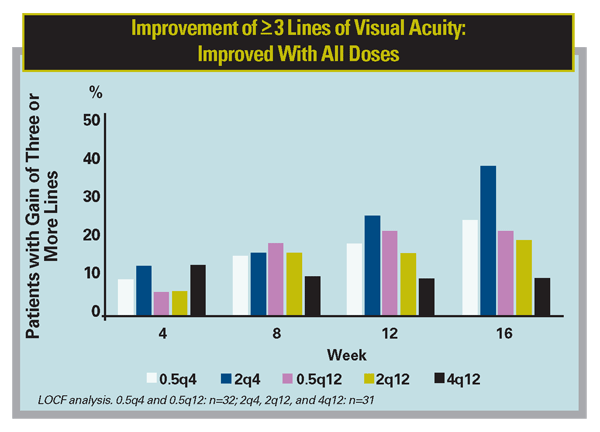
The ophthalmic community eagerly looks forward to agents that may simplify the frequent intravitreal dosing regimen currently representing the standard paradigm for exudative AMD treatment. VEGF Trap may represent such an agent.
Dr. Ciulla is on the Retina Service at Midwest Eye Institute and is an attending physician and surgeon at
Selected
1. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. PNAS 2002;99:11393-8.
2. Saishin Y, Saishin Y, Takahashi K, et al. VEGF-TRAP R1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol 2003;195:241-8.
3. Regeneron Announces Positive Primary Endpoint Results from a Phase 2 Study of VEGF Trap-Eye in Age-related Macular Degeneration. [online]. Available at: http://www.regeneron.com/company/ press_detail.asp?v_c_id=283.



