To estimate the number of individuals in the United States who may benefit from treatment with ranibizumab to treat neovascular AMD and prevent AMD-related blindness, Neil M. Bressler, MD, of Wilmer Eye Institute at Johns Hopkins University and colleagues designed a modeling study using outcomes from three previous Phase III ranibizumab trials.
Using statistics from the Beaver Dam Eye Study and data from the 2008 U.S. Census Bureau, the model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Using data from the Age-Related Eye Disease Study, the authors estimated that one-third of these cases (51,000 individuals) would already have preexisting choroidal neovascularization in the opposite eye, making those patients ineligible for the model used in this study. Of the 151,340 individuals, the authors estimated that ranibizumab would be accessible to 103,582 individuals, making them eligible for inclusion in the study’s modeling criteria.
Based on the model designed for the study, if no treatment were given to the 103,582 cases for which monthly ranibizumab was indicated and accessible, 16,268 (16 percent) would progress to legal blindness in two years. The authors estimated that monthly ranibizumab usage would reduce the incidence of legal blindness in two years by 72 percent, to 4,484 individuals. Additionally, based on the model designed for the study, if no treatment were applied to the 103,582 cases for which monthly ranibizumab is indicated and accessible, 34,702 (34 percent) would progress in two years to visual impairment (worse than 20/40 in the better-seeing eye, a level that precludes an unrestricted driver’s license in most states). The authors estimated that monthly ranibizumab usage would reduce the incidence of visual impairment in two years by 37 percent, to 21,919 individuals.
Based on results of the model designed for this study, the authors conclude that ranibizumab would have an effect on reducing the occurrence of visual blindness in individuals with AMD when treatment is administered on a monthly basis when available.
 |
New research discovery published in the Journal of Leukocyte Biology (
jleukbio.org) offers hope for new Drugs that treat the cellular cause
of the dry-eye disease rather than its symptoms. That’s because the
research is the first to identify natural killer cells, a type of cell
that provides innate immunity to the eyes, as promoting the inflammation
that plays a critical role in the development of dry-eye disease.
“Dry-eye disease is suffered by millions of people in the U.S. but still lacks effective management,” said Yihe Chen, MD, a researcher involved in the work from the Schepens Eye Research Institute at the Massachusetts Eye and Ear Infirmary of the Department of Ophthalmology at Harvard Medical School in Boston.
“Our study has promoted the further understanding of the pathogenesis of dry-eye disease, which is fundamental to develop new treatments and thus improve quality of life for those with this disease.”
To make their discovery, the scientists tested two groups of mice. The first group was normal and the second group was depleted of natural killer cells. When both sets of mice were induced with dry-eye disease under the same conditions, the disease was less severe in the mice depleted of NK cells than the normal mice. This suggests that NK cells play a pivotal role in the development and severity of the disease, making them a target for the development of new drugs.
Progress in Using Stem Cells to Reverse Blindness
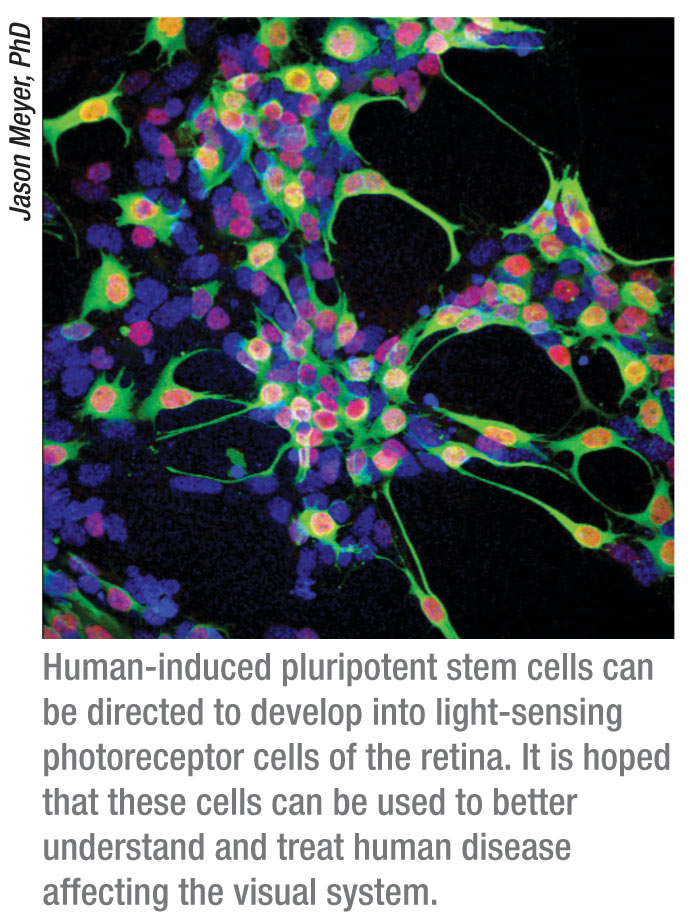
Researchers have used cutting-edge stem cell technology to correct a genetic defect present in a rare blinding disorder, another step on a promising path that may one day lead to therapies to reverse blindness caused by common retinal diseases like macular degeneration and retinitis pigmentosa.
In a study appearing in an advance online publication of the journal Stem Cells on June 15, 2011, investigators used recently developed technology to generate induced pluripotent stem, or iPS, cells from a human patient with an uncommon inherited eye disease known as gyrate atrophy. This disorder affects retinal pigment epithelium cells, the cells critical to the support of the retina’s photoreceptor cells, which function in the transmission of messages from the retina to parts of the brain that interpret images.
 “When we generate iPS cells, correct the gene defect that is responsible
for this disease, and guide these stem cells to become RPE cells, these
RPE cells functioned normally. This is exciting because it
demonstrates we can fix something that is out of order. It also
supports our belief that in the future, one might be able to use this
approach for replacement of cells lost or malfunctioning due to other
more common diseases of the retina,” said lead study author cell
biologist
Jason Meyer, PhD, assistant professor of biology in the School
of Science at Indiana University-Purdue University Indianapolis.
“When we generate iPS cells, correct the gene defect that is responsible
for this disease, and guide these stem cells to become RPE cells, these
RPE cells functioned normally. This is exciting because it
demonstrates we can fix something that is out of order. It also
supports our belief that in the future, one might be able to use this
approach for replacement of cells lost or malfunctioning due to other
more common diseases of the retina,” said lead study author cell
biologist
Jason Meyer, PhD, assistant professor of biology in the School
of Science at Indiana University-Purdue University Indianapolis.
Because iPS cells can be derived from the specific patient who needs Them, use of these cells may avoid the problem of transplant rejection. In the study, vitamin B-6 also was used to treat the damaged RPE cells producing healthy cells that functioned normally. The retina is a relatively easily accessible part of the central nervous system, which makes it an attractive target for correction with iPS cells. Researchers are hopeful that once the gene defect responsible for a blinding disorder is corrected in iPS cells, these cells may be able to restore vision.
Next Generation Gene Therapy
Inspired by earlier successes using gene therapy to correct an inherited type of blindness, investigators from the University of Pennsylvania are poised to extend their approach to other types of blinding disorders.
In a previous human trial conducted at the Children’s Hospital of Philadelphia and Penn, researchers packaged a normal version of a gene missing in Leber’s congenital amaurosis inside a genetically engineered vector, called an adeno-associated virus (AAV). The vector delivered the gene to cells in the retina, where the gene produces an enzyme that restores light receptors.
The results from three Phase I clinical trials for LCA showed the potential for gene therapy based on adeno-associated viruses delivering corrective genes to the retina,” notes co-senior author Jean Bennett, MD, PhD, the F.M. Kirby professor of ophthalmology. “To broaden treating inherited eye diseases, we will need a larger vector toolkit, and what we have seen of AAV8 gives us hope for creating gene therapies for diseases that attack the retina’s photoreceptors. This preclinical study provides the guidance we need to formulate dose level and type of vector to deliver corrective genes to treat blindness caused by the loss of photoreceptors.”
In the present study, published in Science Translational Medicine, the
Penn team compared the safety and efficiency of delivery in an animal
model of two different types of AAVs—AAV2, which was used in the human
trials for LCA, and AAV8, a second-generation AAV technology initially
discovered in the lab of co-senior author
James M. Wilson, MD, PhD,
professor of pathology and laboratory medicine.
“Dry-eye disease is suffered by millions of people in the U.S. but still lacks effective management,” said Yihe Chen, MD, a researcher involved in the work from the Schepens Eye Research Institute at the Massachusetts Eye and Ear Infirmary of the Department of Ophthalmology at Harvard Medical School in Boston.
“Our study has promoted the further understanding of the pathogenesis of dry-eye disease, which is fundamental to develop new treatments and thus improve quality of life for those with this disease.”
To make their discovery, the scientists tested two groups of mice. The first group was normal and the second group was depleted of natural killer cells. When both sets of mice were induced with dry-eye disease under the same conditions, the disease was less severe in the mice depleted of NK cells than the normal mice. This suggests that NK cells play a pivotal role in the development and severity of the disease, making them a target for the development of new drugs.
Progress in Using Stem Cells to Reverse Blindness
Researchers have used cutting-edge stem cell technology to correct a genetic defect present in a rare blinding disorder, another step on a promising path that may one day lead to therapies to reverse blindness caused by common retinal diseases like macular degeneration and retinitis pigmentosa.
In a study appearing in an advance online publication of the journal Stem Cells on June 15, 2011, investigators used recently developed technology to generate induced pluripotent stem, or iPS, cells from a human patient with an uncommon inherited eye disease known as gyrate atrophy. This disorder affects retinal pigment epithelium cells, the cells critical to the support of the retina’s photoreceptor cells, which function in the transmission of messages from the retina to parts of the brain that interpret images.
 “When we generate iPS cells, correct the gene defect that is responsible
for this disease, and guide these stem cells to become RPE cells, these
RPE cells functioned normally. This is exciting because it
demonstrates we can fix something that is out of order. It also
supports our belief that in the future, one might be able to use this
approach for replacement of cells lost or malfunctioning due to other
more common diseases of the retina,” said lead study author cell
biologist
Jason Meyer, PhD, assistant professor of biology in the School
of Science at Indiana University-Purdue University Indianapolis.
“When we generate iPS cells, correct the gene defect that is responsible
for this disease, and guide these stem cells to become RPE cells, these
RPE cells functioned normally. This is exciting because it
demonstrates we can fix something that is out of order. It also
supports our belief that in the future, one might be able to use this
approach for replacement of cells lost or malfunctioning due to other
more common diseases of the retina,” said lead study author cell
biologist
Jason Meyer, PhD, assistant professor of biology in the School
of Science at Indiana University-Purdue University Indianapolis.
Because iPS cells can be derived from the specific patient who needs Them, use of these cells may avoid the problem of transplant rejection. In the study, vitamin B-6 also was used to treat the damaged RPE cells producing healthy cells that functioned normally. The retina is a relatively easily accessible part of the central nervous system, which makes it an attractive target for correction with iPS cells. Researchers are hopeful that once the gene defect responsible for a blinding disorder is corrected in iPS cells, these cells may be able to restore vision.
Next Generation Gene Therapy
Inspired by earlier successes using gene therapy to correct an inherited type of blindness, investigators from the University of Pennsylvania are poised to extend their approach to other types of blinding disorders.
In a previous human trial conducted at the Children’s Hospital of Philadelphia and Penn, researchers packaged a normal version of a gene missing in Leber’s congenital amaurosis inside a genetically engineered vector, called an adeno-associated virus (AAV). The vector delivered the gene to cells in the retina, where the gene produces an enzyme that restores light receptors.
The results from three Phase I clinical trials for LCA showed the potential for gene therapy based on adeno-associated viruses delivering corrective genes to the retina,” notes co-senior author Jean Bennett, MD, PhD, the F.M. Kirby professor of ophthalmology. “To broaden treating inherited eye diseases, we will need a larger vector toolkit, and what we have seen of AAV8 gives us hope for creating gene therapies for diseases that attack the retina’s photoreceptors. This preclinical study provides the guidance we need to formulate dose level and type of vector to deliver corrective genes to treat blindness caused by the loss of photoreceptors.”
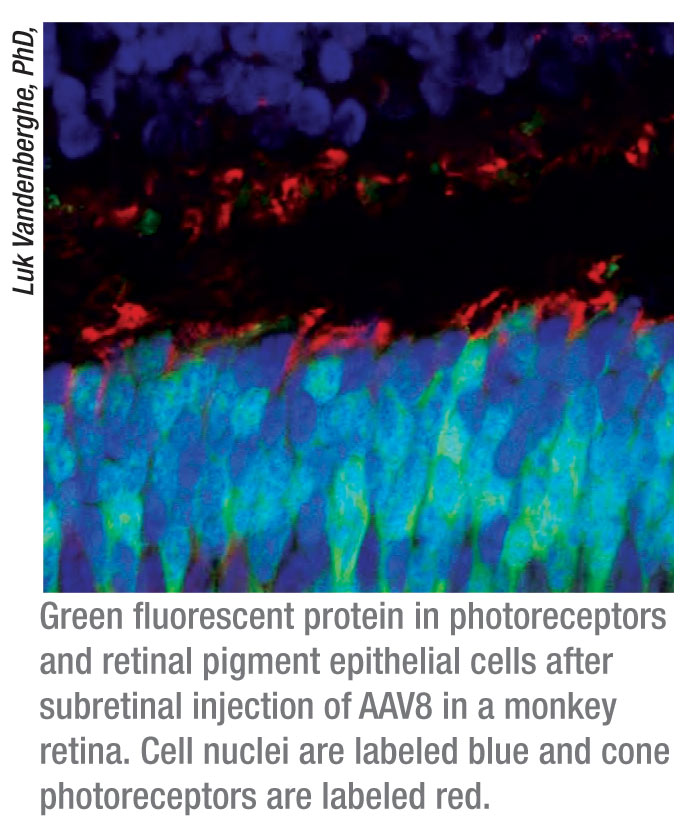 |
The researchers used both vectors to deliver a green fluorescent protein
transgene to the retinal pigment epithelial cells and photoreceptor
cells of nonhuman primates.
“We showed that we can use AAV8 to deliver genes to the photoreceptor of the primate eye at lower doses, both safely and efficiently,” says first author Luk H. Vandenberghe, PhD, senior investigator.
Both AAV2 and AAV8 delivered the gene safely and efficiently to the monkey retinas, but AAV8 was markedly better at targeting photoreceptor cells.
The STM study describes the dose relationship between AAV2 and AAV8 vectors and their target cells and the immune response in the nonhuman primate retina. From this the researchers found dosage thresholds To safely and efficiently target cells in the outer retina such as RPE cells and rod and cone photoreceptors. While AAV2 and AAV8 efficiently delivered the gene to RPE cells at moderate to low doses, expression of the GFP gene in rod and cone photoreceptors was reached only at higher dosages. Substantial delivery to rods was obtained with moderate doses of AAV8, doses similar to those currently used in experimental clinical protocols.
The vectors at intermediate doses did not cause problematic immune responses and post-surgical injection complications. The delivered gene also stayed in the retina at very high levels throughout the study duration of four months. Additionally, the GFP gene was preferentially transduced to one type of retinal ganglion cells, which surprised the researchers. In general retinal ganglion cells transmit visual information from the retina to several regions of the brain. This knowledge could be used in the future to further delineate the neuronal connections between the retina and the brain.
In earlier animal studies, AAV8 also delivered genes safely and efficiently to the mouse retina. However, the mouse retina differs significantly from the primate retina, most notably in structure, which affects the surgical approach to delivering corrective genes to different parts of the eye. The present study in nonhuman primates is the next step to better translate treatment strategies for people.
“To address patients with other retinal diseases, we need a renaissance of technology—new and better vectors to safely and effectively deliver corrective genes to a range of diseases,” says Dr. Wilson. “My lab has recently isolated new families of simian-based adenoviruses and adeno-associated viruses. Recombinant versions of these viruses are turning out to be useful as improved gene transfer vehicles to a variety of targets
“We showed that we can use AAV8 to deliver genes to the photoreceptor of the primate eye at lower doses, both safely and efficiently,” says first author Luk H. Vandenberghe, PhD, senior investigator.
Both AAV2 and AAV8 delivered the gene safely and efficiently to the monkey retinas, but AAV8 was markedly better at targeting photoreceptor cells.
The STM study describes the dose relationship between AAV2 and AAV8 vectors and their target cells and the immune response in the nonhuman primate retina. From this the researchers found dosage thresholds To safely and efficiently target cells in the outer retina such as RPE cells and rod and cone photoreceptors. While AAV2 and AAV8 efficiently delivered the gene to RPE cells at moderate to low doses, expression of the GFP gene in rod and cone photoreceptors was reached only at higher dosages. Substantial delivery to rods was obtained with moderate doses of AAV8, doses similar to those currently used in experimental clinical protocols.
The vectors at intermediate doses did not cause problematic immune responses and post-surgical injection complications. The delivered gene also stayed in the retina at very high levels throughout the study duration of four months. Additionally, the GFP gene was preferentially transduced to one type of retinal ganglion cells, which surprised the researchers. In general retinal ganglion cells transmit visual information from the retina to several regions of the brain. This knowledge could be used in the future to further delineate the neuronal connections between the retina and the brain.
In earlier animal studies, AAV8 also delivered genes safely and efficiently to the mouse retina. However, the mouse retina differs significantly from the primate retina, most notably in structure, which affects the surgical approach to delivering corrective genes to different parts of the eye. The present study in nonhuman primates is the next step to better translate treatment strategies for people.
“To address patients with other retinal diseases, we need a renaissance of technology—new and better vectors to safely and effectively deliver corrective genes to a range of diseases,” says Dr. Wilson. “My lab has recently isolated new families of simian-based adenoviruses and adeno-associated viruses. Recombinant versions of these viruses are turning out to be useful as improved gene transfer vehicles to a variety of targets



