All LASIK surgeons are familiar with the basic list of concerns to address when screening candidates for LASIK, including dry eye, potential keratoconus, vascular disease, diabetes, herpes, rosacea, blepharitis, corneal dystrophies and early cataract. However, surgeons often come up with helpful strategies for screening patients that are not widely known. With that in mind, we asked some experienced surgeons to share insights and strategies that help them avoid unfortunate LASIK outcomes.
Looking for Keratoconus
Every LASIK surgeon wants to avoid operating on a patient who is likely to develop keratoconus, but finding those patients can be tricky. Our surgeons offered these helpful strategies:
• Use the right scale when checking topographic maps. According to James J. Salz, MD, clinical professor of ophthalmology at the University of Southern California, reading corneal topography maps isn't just a question of knowing what to look for. Dr. Salz notes that these maps can be generated at different scales, and using some scales can cause the data to be translated in a way that's misleading.
"Although it's counter-intuitive," he explains, "less detail is actually better when you're looking at these abnormalities." As an example, he shows a topographic scan of one of his pre-LASIK patients generated at three different scales. (See images, below.) "The first map indicates a definite asymmetry inferiorly," he says. "However, the color scale runs from 40 to 45 D, so all those colors represent a 5-D spread. That's almost a quarter-diopter scale. The sensitivity is so great that it accentuates subtle abnormalities and makes them look grossly abnormal.
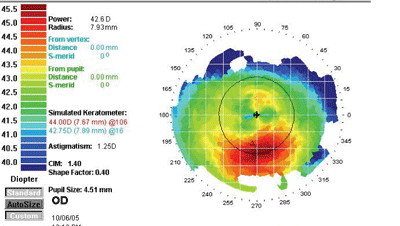 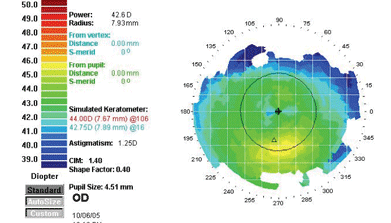 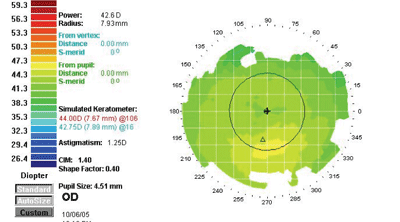 |
| The scale of a topographic map can cause the data to be misinterpreted. These maps were produced from the same data, displayed at different scales. The left map color scale covers only 40 to 45 D; the middle map covers 39 to 50 D; the right map, 26 to 59 D, about 1.5 D per color. The last is the scale originally recommended by Stephen D. Klyce, PhD, creator of this data presentation format. James J. Salz, MD |
"With each enlargement of the scale, the red area down below starts looking less abnormal," he continues. "In the second version of the map, the scale goes from 39 D to 50 D in about half-diopter stops. Even though the map looks a little suspicious—note the bright yellow area—it doesn't look as abnormal as the first map.
"On the last map, the scale goes from 26 D to 59 D," he notes. "At this scale, with about 1.5 D to a color, the map looks fairly normal. Comparing it to the others, you wouldn't know it was the same eye. In fact, the data has simply been processed differently." Dr. Salz adds that the final 1.5 D scale was recommended by Stephen D. Klyce, PhD, at Louisiana State University, when he first conceived of presenting topography data with color coding to represent flat versus steep areas of corneal curvature.
"When we're screening patients," he concludes, "we don't want to exclude people who are probably normal."
• Ask about a family history of corneal problems in the simplest possible terms. Scott MacRae, MD, professor of ophthalmology and professor of visual science at the University of Rochester in New York, notes that determining which patients are likely to develop keratoconus is challenging, despite all the tests that are available. "There's a small group of people in the population who will eventually develop keratoectasia because they have a genetic predisposition," he says. "It can be impossible to detect because the disease is in its latent stage and we don't currently have the genetic tests to detect it.
"Even with the best screening techniques, occasionally patients who probably shouldn't be treated get treated," he continues. "For example, I had one patient with about 2 D of regular astigmatism and normal topographies who was already in the OR. We'd previously asked her about a family history of keratoconus, and she'd said no. We were talking about her high level of astigmatism when she mentioned that her sister also had high levels of astigmatism; then she added that when the sister had undergone further testing, they found corneal disease. When I heard that, I promptly cancelled the surgery!
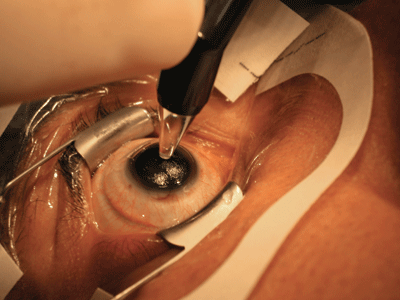 |
| Measuring the stromal bed in the OR after the flap is retracted ensures that sufficient tissue is left, and makes it possible for you to calculate the average flap depth and variance your keratome is producing. Steve C. Schallhorn, MD |
"We later found out that the sister had keratoconus," he says. "So the patient had a positive family history of keratoconus but didn't know it. Unfortunately, that's not uncommon, because most people don't know what keratoconus is."
• Try these four tests when topography is suspicious. Dr. Salz describes four additional tests he performs when faced with abnormal topography:
1) Differential pachymetry. This is the term Dr. Salz uses for measuring corneal thickness both centrally and inferiorly. "The cornea should be thicker inferiorly than it is centrally," he explains. "If it isn't, I'd be concerned."
2) Dilated retinoscopy. "If a patient has early signs of keratoconus, the light reflex will be a scissoring reflex," he explains.
3) Look for sharp mires. Dr. Salz observes that when doing manual keratometry, irregular mires can be an early and very subtle sign of irregular astigmatism, often present in early keratoconus.
4) Check refraction stability. "If a patient had a 0.5 D of astigmatism a year ago and now has 1.5 D and inferior steepening, I'd be very concerned that she might be developing a cone," says Dr. Salz.
Leaving Sufficient Stromal Tissue
Steve C. Schallhorn, MD, director of refractive surgery for the U.S. Navy, who runs the refractive surgery program at the Navy Medical Center in San Diego, says one of the hot issues right now is accurately estimating the amount of tissue that will be left in the stromal bed after surgery. "The object, of course, is to try to reduce the chance of ectasia," he says. "The historic standard is to have 250 µm remaining, but there are many surgeons who aim for a greater number. My current minimum is 270 µm; some use 300 µm or more as the lower limit.
"Today, the best way to accurately determine residual stromal bed thickness is by doing pachymetry in the OR after the flap is lifted," says Dr. Schallhorn, noting that this isn't commonly done. "This ensures that you have enough tissue to proceed safely. The problem is that it's difficult to predict the actual thickness of most keratome cuts. Of course, practically speaking, you also need to be able to estimate what will be left even before you decide to proceed with surgery, but doing pachymetry when the patient is on the table is a key part of that process as well."
To estimate the amount of tissue that will remain following an ablation, a number of factors must be determined: first, the preop thickness of the cornea; second, the thickness of the flap; and third, the estimated depth of the ablation. "Knowing the thickness of the flap made by whatever keratome you're using is very important," says Dr. Schallhorn. "Keratomes often don't cut the depth marked on the cutting head, and a given keratome may cut differently in the hands of different surgeons."
Dr. Schallhorn says the way to accomplish this is to measure corneal thickness before the flap is cut, and then measure stromal thickness in the OR after the flap has been retracted. Subtracting the two numbers gives a more accurate measure of flap thickness. "If you do that in enough patients, you can derive the average flap thickness as well as the variance around that average," he notes.
"For example, suppose you use a keratome with a 160-µm cutting head," he says. "After many measurements, say you find that the flaps, on average, are 120 µm, with a standard deviation of 20 µm. For a preoperative workup, add two standard deviations to the 120 µm average, for a total of 160 µm, and use this as a conservative flap thickness for computation purposes. Approximately 95 percent of flaps should be less than this thickness. You can then calculate the estimated residual bed by subtracting this number, as well as the maximum ablation depth, from the central corneal thickness.
"Using 160 µm instead of the 120-µm average," he continues, "may help prevent a surprise in the OR. If you use the 120-µm average in the computation but then happen to create a much thicker flap, the residual bed could be close to the minimum." He adds that in this situation you might have been better off steering the patient to a surface procedure right from the start.
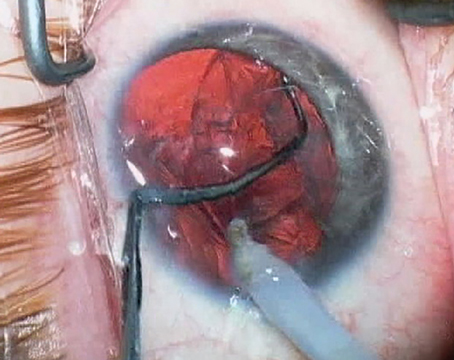
Dr. Schallhorn notes that newer devices, such as the Zeiss Visante and the Oculus Pentacam (with its high-resolution head), can directly image the postoperative corneal flap interface and obtain a precise measurement of flap thickness and the residual bed. (See image, facing page.) "This is very useful for retreatments," he says. "You can determine whether there's sufficient tissue left for an enhancement by subtracting the estimated ablation depth from the measured stromal bed."
Avoiding Pupil Pitfalls
Recently, a St. Louis County jury awarded a LASIK patient more than $3 million in damages on the grounds that the surgeon failed to measure his pupil size properly and failed to warn him of the possible negative consequences of his larger-than-average pupils. The patient claimed that he sustained "significant and permanent disturbances in his vision, particularly at night."
"Everybody agrees that we should measure pupil size," notes Dr. Salz. "There are many ways to do this; I like the NeurOptics Pupillometer, which is fast and objective. But whatever technique you use, make sure you measure the pupil in the dark. Illuminating the eye constricts the pupil and produces an accurate measurement."
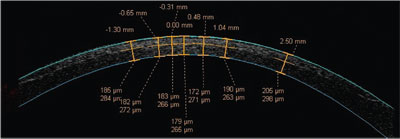 |
| A high-resolution scan of the cornea post-LASIK using optical coherence tomography (Visante, Carl Zeiss Meditec) shows the flap interface, and can be used to determine flap and residual stromal bed thickness. Numbers above the cornea show distance from the corneal center in millimeters. Paired numbers below show flap and residual bed thickness in microns. Carl Zeiss Meditec |
Based on his experience, Dr. Salz offers the following strategies for creating happier patients and reducing the risk of lawsuits:
• Don't tell patients pupil size doesn't matter. Dr. Salz notes that two recent studies found that large-pupil patients had the same incidence of night glare, halos and night vision complaints as patients with smaller pupils. Because of these studies, he says, some surgeons are telling their patients that pupil size is not a risk factor for night vision problems. "These studies were well done, by reliable investigators," he says, "but they were done with specific lasers, one of them using an algorithm not available in the United States. You can't assume that your laser will produce the same results. There are other studies that give us good reason to see pupil size as a risk factor—not to mention the recent $3 million award."
Dr. Salz notes that numerous studies have shown that higher-order aberrations increase with pupil size. "For example," he says, "consider the wavefront analysis of one of my preop patients, who had the largest scotopic pupils I've encountered to date—at times over 9 mm. When her left pupil diameter was 8 mm, her spherical aberration was .21 and her coma was .52. When she was dilated to 9.7 mm—close to her natural scotopic pupil size
—her spherical aberration more than doubled to .43, and coma reached 1.36.
"You also have to consider that even custom LASIK will probably make these numbers worse," he adds. "With this patient we did extensive informed consent, showing her the relationship between pupil size and higher-order aberrations, and explaining that the surgery might make the aberrations worse. Because she was highly motivated, she chose to proceed with the first eye. Postop, with the pupil at 8.6, her spherical aberration rose to 2.2—10 times her preop 8-mm number.
"The bottom line is if you tell a patient he's not at risk because of his pupil size, regardless of your justification, he may later go to someone else," he says. "If that doctor makes his pupil smaller and his symptoms go away, how can you argue that the pupil was not important? In addition, the patient brochures for custom surgery from Visx and Alcon both warn that patients are at risk of vision problems if they have larger-than-normal pupils. Even the FDA website contains a similar warning. So not telling the patient that he's at risk unquestionably puts you at risk."
Dan Tran, MD, medical director for Coastal Vision Laser Eye Center in Newport Beach, Calif., agrees. "Pupil size does matter, for two reasons," he says. "First, if the pupil is large and the treatment area is large, you're not going to end up with a prolate cornea. There's no way you can maintain a prolate shape under those conditions, even with wavefront. That means you're going to increase spherical aberration. And the greater the correction, the more significant the problem.
"Second," he continues, "the treatment must be perfectly centered, taking into account not only the pupil, but also the visual axis. A slight decentration will cause problems if the pupil is large, and the larger the pupil size, the more likely it is that you'll get into trouble."
• Do one eye and see how the patient reacts. "I do one eye at a time in everybody," says Dr. Salz, "but particularly in a large pupil patient. That way I don't do the second eye until I'm sure that they're satisfied with their night vision."
• Pretest with Alphagan. If a patient has large pupils, Dr. Salz suggests making sure that eye drops will be an option for countering dilation at night. "Let the patient know that he might need to use the drops for the rest of his life to minimize night vision problems," he says.
• For large pupils, do preop wavefront analysis. If the pupil is bigger than 6.5 mm, do a wavefront study and see how high the preop wavefronts scores are. If a patient has a relatively low level of aberrations, he'll probably be fine.
• If possible, perform custom LASIK. "Conventional surgery tends to double preop higher-order aberrations," observes Dr. Salz. "If the patient starts with .5 RMS and you do conventional LASIK, you may double that to 1.0, and the patient will probably have a lot of trouble.
"In contrast," he continues, "custom surgery doesn't generally increase these aberrations as much as conventional LASIK." Dr. Salz says this has been borne out by his clinical experience. "Since we've begun performing wavefront-based treatments, I haven't had a single patient—even the patient with the 9-mm pupil—choose not to have the second eye done. That wasn't true when I was doing conventional surgery. Some of those patients have never done their second eye because they were so freaked out by the quality of their night vision."
|
Checking for Loose Epithelium
When Dr. Tran suspects there may be a problem with the epithelium, he uses a wet Q-Tip to check its stability. "Patients with some conditions tend to have a loose epithelium after LASIK," he explains. "That can delay healing and cause problems with visual recovery and refractive outcomes. So, if I see evidence of basement membrane dystrophy or previous scarring, or the patient has a history of recurrent corneal erosions or diabetes, or has pain or a decrease in vision, I anesthetize the cornea and try to gently move the epithelium with the Q-Tip. If the epithelium is loose, it will slide a little bit. In some cases, you can even see it move."
Dr. Tran says a positive Q-Tip test confirms a decision to avoid LASIK, whereas a negative result—no movement—simply tells him to base his judgment on other factors. "If the epithelium is loose, we may end up doing PRK instead of LASIK," he says. "If it isn't loose, my decision will depend on other contrary indications. For example, if a patient has a history of recurrent erosions or I see evidence of anterior basement membrane dystrophy, I probably will still switch to PRK."
Dr. Tran does note a certain irony to the situation. "The potential problem with a loose epithelium before LASIK is a delay in healing, but there's no way to be certain whether it will happen. And if we switch to PRK, the patient will have a delayed visual recovery anyway."
Dry Eyes, Allergies and Inflammation
To help minimize problems resulting from these causes:
• Don't rely on Schirmer strips. "We've looked at Schirmer strips and found them to be of little value in this situation," notes Dr. Schallhorn. "They're not a very sensitive test for detecting who will have dry-eye problems after LASIK, because they only measure aqueous production—not the two other critical layers of the tear film: mucin and oil. As a result, a patient can have dry-eye symptoms but still have a robust Schirmer's, or have no symptoms or signs despite a relatively low Schirmer's. Patients who have blepharitis or meibomian gland dysfunction often have normal Schirmer's. At the same time, if the test finds almost no wetting, the patient will likely have signs and symptoms of dry eye that can be picked up on a slit lamp exam without using the Schirmer test."