Our understanding of macular hole formation and treatment has advanced tremendously over the years, but remains incomplete. The application of high resolution imaging technology is helping to corroborate theories of pathogenesis as well as providing valuable new information. This article reviews the latest theories of macular hole pathogenesis, approaches to macular hole repair, and outcomes and complications of vitreous surgery for macular hole.
Macular Hole Pathogenesis
The exact cause of macular hole remains unknown. The first reported macular holes in the late 19th century were believed to result from trauma that caused cystoid changes in the macula. Concurrent with the discovery around 1970 that the majority of macular holes were not associated with trauma, the predominant thought was that macular hole etiology was related to the presence of cystoid macular edema (CME), including any condition that may lead to CME.1
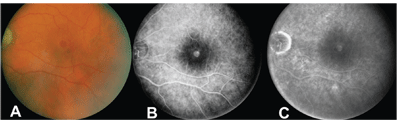
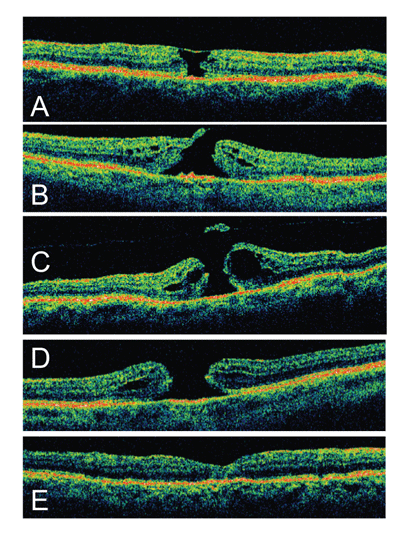
The vitreomacular traction theory of macular hole pathogenesis gained in popularity with the recognition that peripheral retinal breaks occur secondary to vitreoretinal traction and that strong adhesion exists between the vitreous and fovea. The recognition of a temporal association between posterior vitreous detachment (PVD) and full-thickness macular hole assisted J. Donald M. Gass, MD, in the development of his grading scheme.2 Optical coherence tomography has enhanced our understanding of the orientation of this traction, which is now thought to be predominantly antero-posterior (A-P) in direction.
|
The natural history of macular hole is that of deterioration over a few years with subsequent stabilization of both visual acuity and hole size. In a study of 198 patients with untreated macular hole, about one-third of patients had an increase in size of the hole and almost half experienced a decrease in visual acuity of at least two lines. Approximately 7 percent of patients developed a macular hole in the fellow eye.5
Surgery for Macular Hole
Introduced about 15 years ago, surgery for macular hole including vitrectomy, fluid-gas exchange and face-down postoperative positioning initially had an anatomic success rate of 58 percent.6 Since that time, macular hole surgery has been performed on thousands of patients and a greater than 90 percent closure rate is currently expected after one surgery.7 Vision improvement is noted in the majority of patients with hole closure, with vision better than 20/40 in 27 percent to 71 percent.8 Level 1 evidence has supported surgery for stage 2 macular hole to prevent decreased visual acuity and progression to later stages.9 Surgery has also been shown to improve visual acuity in the majority of patients with stage 3 and 4 macular hole.8
Despite apparent anatomic success after macular hole surgery, some patients still do not achieve the expected improvement in visual acuity. Histopathologic evidence has shown that the majority of opercula removed from eyes with macular hole do not contain neuronal elements. Interestingly, however, one study revealed that cases in which opercula do contain neuroretinal tissue do not have significant postoperative improvement in visual acuity.10 This raised the possibility that the degree of foveal damage is determined at the time of macular hole formation, and the potential for surgical success may be limited if extensive retinal damage is present.
Recent use of ultrahigh-resolution optical coherence tomography has enabled visualization of persistent retinal abnormalities that likely would not have been recognized without modern imaging technology.11 These abnormalities may be in part responsible for lack of improvement in visual acuity in eyes after macular hole surgery. It is also possible that newer surgical techniques, including internal limiting membrane peeling and staining, may be in part responsible for retinal damage and decreased visual outcomes despite greater rates of anatomic closure.
|
Possible mechanisms to explain why vitrectomy and fluid-gas exchange can close a macular hole include relief of traction (tangential and A-P) and stimulation of fibroglial proliferation to plug the hole. Histopathologic evidence has revealed hyperplastic RPE and fibroglial cells associated with spontaneously closed macular holes.12 A variety of adjuvant therapies have been used at the time of surgery in an effort to enhance glial proliferation. Prospective, randomized studies of transforming growth factor beta (TGF-ß) and autologous platelets have, however, shown little benefit in terms of anatomic closure or final visual acuity.13,14
Peeling of epiretinal (ERM) or internal limiting membrane (ILM), through release of cytokines, is thought to induce a similar fibroglial proliferation in order to facilitate macular hole closure. ILM peeling may also remove any contractile ERM formed above it, thereby relieving tangential traction. In addition, membrane removal can enhance mobility of the hole edges, thereby facilitating re-approximation.
One retrospective study suggests that ILM peeling is beneficial in attaining improved visual acuity and anatomic closure in macular holes of less than six-months duration.15 A multicenter, controlled randomized trial showed a significant increase in closure rates with ERM peeling, but no effect on visual acuity.16 Accordingly, there has been some concern regarding damage to the retina secondary to membrane peeling. Another study showed that internal limiting membrane peeling at the time of macular hole surgery significantly improves the closure rate of macular holes larger than 400 µm in diameter but has no effect on the closure rate of smaller macular holes.17 Based on an association between ERM formation and late hole reopening, some have postulated that ILM peeling may prevent macular hole recurrence.
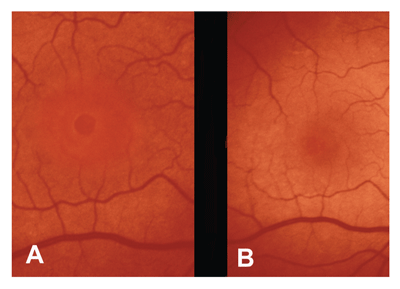
One study using scanning laser ophthalmoscopy revealed paracentral scotomas in more than half of patients after ILM peeling.18 However, a prospective study with five-year follow-up revealed that macular hole surgery with ILM peeling is a safe and effective procedure with 95 percent of patients achieving anatomic closure and 92 percent achieving improvement in visual acuity.19 In that study, the majority of patients (97 percent) were pseudophakic at five years, but there were no cases of late hole reopening or ERM formation after successful hole closure. The absence of a large randomized clinical trial likely accounts for the lack of consensus regarding the efficacy of and indications for ILM peeling.
|
Infusion of silicone oil or a gas bubble theoretically provides a scaffolding or template for fibroglial proliferation. Face-down positioning is commonly used to facilitate macular hole tamponade and closure postoperatively. Long-acting gas with face down positioning for up to three weeks was used earlier in the development of surgery for macular hole. There is evidence, however, that macular holes can be effectively closed without strict positioning regimens. One study of stage 3 and 4 macular holes revealed a 90 percent closure rate with significant improvement in visual acuity one year after vitrectomy combined with ILM peeling, instillation of C3F8 gas, and 15 day postoperative period avoiding the supine position. Accordingly, OCT has demonstrated anatomic resolution beginning as soon as one day after surgery.21
It has been suggested that silicone oil may improve the tamponade effect and decrease the need for face-down positioning; however, there is evidence that success rates with gas are superior. One study demonstrated greater one-surgery anatomic success and superior visual acuity with gas tamponade compared to silicone oil.22
Surgical Complications
With improving surgical techniques, we have seen a decline in complication rates with macular hole surgery. A review of three multicenter, randomized, controlled trials found the following complications to be most prevalent: cataract (>75 percent), late macular hole reopening (2 percent to 10 percent), retinal detachment (3 percent), and endophthalmitis (<1 percent).8 Because the rate of cataract formation is so high, some surgeons remove the lens prior to or simultaneous with the macular hole surgery. A study of 631 eyes revealed a high rate of peripheral retinal tears, but a significantly lower rate of retinal detachment (2 percent), less than reported in previous studies.23 Visual field defects are another reported complication of macular hole surgery.24 These deficits have been hypothesized to occur secondary to mechanical injury and/or dehydration of the nerve fiber layer during intraoperative air-fluid exchange.
Since macular holes were first recognized in the 19th century, there have been many developments in our understanding and treatment of the condition. This evolution has been facilitated by advancing theories of pathogenesis, improved imaging modalities, including OCT, as well as refinement of surgical technique. Accordingly, outcomes for patients with macular hole have improved dramatically since surgical results were initially reported in 1991. However, many questions remain and are currently being investigated regarding the pathogenesis and optimal surgical techniques for macular hole.
Dr. Wender is an ophthalmology resident at the California Pacific Medical Center (CPMC) in San Francisco. Dr. Jumper is the CPMC Retina Service Chief and is in private practice at the West Coast Retina Medical Group, 185 Berry St., Suite 130, San Francisco, CA 94107. Both can be reached at
wcr@ westcoastretina.com, phone: (415) 972-4600.
1. Smiddy WE, Flynn HW, Jr. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol 2004;137:525-37.
2. Johnson RN, Gass JD. Idiopathic macular holes. Observations, stages of formation, and implications for surgical intervention. Ophthalmology 1988;95:917-24.
3. Lo WR, Hubbard GB. Macular hole formation, spontaneous closure, and recurrence in a previously vitrectomized eye. Am J Ophthalmol 2006;141:962-4.
4. Morgan CM, Schatz H. Idiopathic macular holes. Am J Ophthalmol 1985;99:437-44.
5. Chew EY, Sperduto RD, Hiller R, et al. Clinical course of macular holes: The Eye Disease Case-Control Study. Arch Ophthalmol 1999;117:242-6.
6. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 1991;109:654-9.
7. Williams GA. Macular Holes: The Latest in Current Management. Retina 2006;26:S9-S12.
8. Benson WE, Cruickshanks KC, Fong DS, et al. Surgical management of macular holes: A report by the American Academy of Ophthalmology. Ophthalmology 2001;108: 1328-35.
9. Kim JW, Freeman WR, Azen SP, et al. Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole Study Group. Am J Ophthalmol 1996;121:605-14.
10. Ezra E, Fariss RN, Possin DE, et al. Immunocytochemical characterization of macular hole opercula. Arch Ophthalmol 2001;119:223-31.
11. Ko TH, Witkin AJ, Fujimoto JG, et al. Ultrahigh-resolution optical coherence tomography of surgically closed macular holes. Arch Ophthalmol 2006;124:827-36.
12. Guyer DR, Green WR, De BS, Fine SL. Histopathologic features of idiopathic macular holes and cysts. Ophthalmology 1990;97:1045-51.
13. Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: Results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology 1999;106:932-8.
14. Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor-beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology 1998;105:700-6.
15. Brooks HL, Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000;107:1939-48.
16. Cheng L, Azen SP, El-Bradey MH, et al. Effects of preoperative and postoperative epiretinal membranes on macular hole closure and visual restoration. Ophthalmology 2002;109:1514-20.
17. Tadayoni R, Gaudric A, Haouchine B, Massin P. Relationship between Macular Hole Size and the Potential Benefit of Internal Limiting Membrane Peeling. Br J Ophthalmol 2006.
18. Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol 2001; 132:363-8.
19. Haritoglou C, Reiniger IW, Schaumberger M, et al. Five-year follow-up of macular hole surgery with peeling of the internal limiting membrane: Update of a prospective study. Retina 2006;26:618-22.
20. Mavrofrides E, Smiddy WE, Kitchens JW, et al. Indocyanine green-assisted internal limiting membrane peeling for macular holes: Toxicity? Retina 2006;26:637-44.
21. Jumper JM, Gallemore RP, McCuen BW, Toth CA. Features of macular hole closure in the early postoperative period using optical coherence tomography. Retina 2000;20:232-7.
22. Lai JC, Stinnett SS, McCuen BW. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology 2003; 110:1170-4.
23. Kumagai K, Ogino N. Results of macular hole surgery combined with PEA and IOL. Semin Ophthalmol 2001; 16:144-50.
24. Pendergast SD, McCuen BW. Visual field loss after macular hole surgery. Ophthalmology 1996;103:1069-77.