This year's ARVO offerings in the retina realm center on expanding the indications for treatments we currently use, such as PDT, or acquiring more concrete data on treatments some of us are starting to use more frequently, such as intravitreal injections of triamcinolone acetonide. Here's a look at how researchers are putting these modalities to use, and the results you can expect if you decide to use them in your practice.
Six-month data from a randomized, controlled, prospective study has found no real difference in visual outcomes between PDT alone and PDT in combination with TA injection for occult and minimally classic choroidal neovascularization secondary to age-related macular degeneration. The researchers note, however, that the combination treatment reduces the number of treatments required to render a lesion inactive, and hastens the resolution of macular edema.
In the study, researchers from the Ivey Eye Institute in Ontario equally randomized 20 patients to either combination treatment or PDT alone (control). Over the follow-up period, the primary outcome was the proportion of eyes with fewer than 15 letters of visual acuity loss. Secondary outcomes included the number of PDT treatments, the change in macular thickness, contrast sensitivity, intraocular pressure levels and cataract.
 |
 |
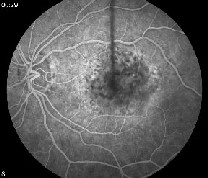
| An AMD patient before (left) and after combination therapy with PDT and Kenalog. Researchers found no effect on visual acuity from the treatment. |
In contrast to this study, a non-comparative report on combined PDT and TA in a larger group of patients by researchers from Germany and Austria found a possible treatment effect.
The physicians prospectively studied 199 patients with CNV from AMD who received intravitreal TA 16 hours after PDT. Eighty percent of the patients had subfoveal lesions, 10 percent had juxtafoveal, and 10 percent had extrafoveal. The average follow-up was 31 weeks. Baseline acuity ranged from 20/100 to hand movement with a mean of 20/125.
There was an average improvement of 1.14 lines of vision at the last follow-up visit. During follow-up, patients required an average of 1.25 treatments to achieve inactivation of the CNV. There was a transient increase in IOP in 38 patients (19 percent). One patient needed surgery for a persistent high IOP.3567
Italian researchers say PDT can be effective in patients with extrafoveal CNV in pathologic myopia, as long as they're younger than 50 years of age. In older patients, the researchers say PDT hardly has an effect.
In a prospective, non-comparative fashion, 24 eyes of 24 patients underwent PDT, and the researchers followed them for two years. Eight patients were younger than 50, and 16 were older. The physicians performed retreatments if there was evidence of CNV leakage on fluorescein. There was an average of 2.91 retreatments (1.75 in patients under 50, 3.5 in those older than 50)—2.25 in the first year, and 0.66 in the second. The baseline acuity ranged from 20/25 to 20/125 in the patients younger than 50, and between 20/25 and 20/80 in those older than 50. At two years, three-quarters of patients under 50 had stable acuity, and a quarter lost at least three lines. In the over-50 group, though, 38 percent had stabilized vision, and 56 percent lost at least three lines.
|
|
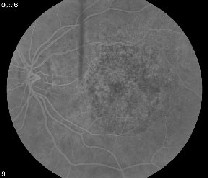 |
| Researchers say PDT can be effective in patients with occult CNV lesions. Here, an eye with occult CNV (left) shows improvement after a year of PDT (right). |
At two years, the foveal avascular zone was involved by the CNV in three out of eight eyes available for follow-up (38 percent) in patients under 50, compared to three-quarters of the eyes in patients over 50. Neither group gained any lines.342
Members of the Verteporfin in Minimally Classic CNV (VIM) Study Group say that minimally classic lesions in PDT-treated eyes are less likely to sustain a three or more line loss of acuity and progress to predominantly classic CNV than placebo-treated eyes at two years. They also note that most lesions progress to predominantly classic CNV within the first six months, making early referral and treatment crucial.
In the study, researchers randomized patients with minimally classic lesions no larger than six MPS disc areas and baseline acuity of 20/250 or better to one of three treatment groups: PDT with reduced fluence (RF group; 300mW/cm2) for 83 seconds; PDT with standard fluence (SF group) for 83 seconds; or placebo followed by sham light. Anyone who progressed to predominantly classic CNV was eligible for open-label verteporfin therapy at a standard fluence.
One hundred three of 117 patients (88 percent) completed the two-year exam. At that time, 23 eyes of 37 given placebo (62 percent) had lost three or more lines, compared with nine of 34 eyes (26 percent) treated with RF and 17 of 32 eyes (53 percent) treated with SF. In the placebo group, 28 percent progressed to predominantly classic CNV. Only 5 percent of the RF group and 3 percent of the SF group developed predominantly classic CNV.
Of the 11 placebo eyes that progressed, seven progressed by month three, one by month six, two by a year and one by two years. Two PDT eyes progressed by the third month, and only one more developed predominantly classic CNV by six months.3566
A group of physicians is investigating the safety and efficacy of the agent squalamine lactate in conjunction with PDT as a treatment for subfoveal CNV associated with AMD. The randomized, controlled trial, now in Phase II, is being sponsored by the Genaera Corp. of Plymouth Meeting, Pa.
The researchers treated 45 patients age 50 and older who qualified for PDT for their CNV (predominantly classic, minimally classic or active occult subtypes). In the treatment groups, physicians infused squalamine lactate intravenously (0.25 mg/mL concentration) once per week at weeks one, two, four, five, nine, 13, 17, 21 and 25. They received PDT in the third week. They could receive additional PDT at week 15 and/or 27 if it was deemed necessary and fit with PDT retreatment criteria.
The parallel treatment groups were:
• Group A: 40 mg over 40 min.;
• Group B: 20 mg over 20 min.;
• Group C: 10 mg over 10 min.;
• Groups D, E and F were controls.
The researchers report no adverse drug interactions, and say that preliminary data suggest that squalamine may be well-tolerated when used with PDT. They will share more detailed safety and efficacy data at the ARVO meeting.2363
Researchers from the University of British Columbia in Vancouver are challenging the belief that PDT is less effective in occult lesions than primarily classic ones.
The physicians conducted a retrospective cohort study of 339 consecutive patients treated with PDT with verteporfin for CNV in AMD, a number that they say exceeds the required sample size of 114 patients per group to achieve a power of 80 percent to detect a minimal clinically significant difference of one line of vision change between groups. They studied patients with occult but no classic lesions (OC) and those with predominantly classic lesions (PC).
PDT treatment, retreatment and follow-up schedules followed the Treatment of AMD with PDT (TAP) guidelines, and the primary outcome was the average change in acuity.
At baseline, 183 eyes (55 percent) had occult-only lesions, with a baseline acuity of 51.2 letters. One hundred fifty-four eyes had predominantly classic lesions, with a baseline vision of 48.1 letters. One hundred thirty-four OC patients (73 percent) and 116 PC patients (74 percent) completed a year of follow-up. The mean loss of letters at that point was 10.6 (PC group) and 10.7 (OC group), yielding no significant difference. At a year, 58 percent of the PC group and 60 percent of the OC group lost no more than 15 letters, which wasn't statistically significant.3572
Intravitreal Steroids
Researchers involved in a randomized, controlled trial of intravitreal injection of TA for diabetic macular edema that's failed laser treatment say that TA's benefit persists for up to two years with retreatments.
In the study, 34 eyes were randomized to receive active treatment of
4 mg TA and 35 received placebo.
 |
 |
| An OCT image of a patient treated with intravitreal triamcinolone injection for macular edema secondary to BRVO before (top) and six months after treatment. |
Adverse events included:
• elevated IOP by 5 mmHg or more in 21/33 TA eyes (64 percent) vs. 3/31 placebo eyes (9 percent);
• 14/33 TA eyes (42 percent) required glaucoma treatment compared to 1/31 placebo eyes (3 percent);
• significant cataract progression in 12/33 TA eyes (36 percent) vs. 3/31 placebo (9 percent);
• 13 of the TA eyes (39 percent) underwent cataract surgery;
• one TA eye developed infectious endophthalmitis that was treated without loss of acuity.4672
A retrospective study from New Jersey examined the potential utility of intravitreal TA injections for macular edema secondary to retinal vein occlusions. They say that multiple injections are necessary for the edema to eventually resolve.
Researchers reviewed 161 eyes of 158 patients who received 1 to 4 mg of intravitreal TA. The edema in 83 of the eyes was secondary to branch retinal vein occlusion, 61 cases were secondary to CRVO, and 17 cases occurred after hemicentral retinal vein occlusion. Forty-eight eyes underwent laser treatment, and 45 had cataract surgery before TA injection. The patients were followed for an average of 10 months. The results at the three- and six-month time points appear in Tables 1 and 2. Forty-four eyes required laser treatment, and 65 needed at least one additional TA injection during follow-up.4042
|
Table 1. Visual Results of Kenalog for Vein Occlusion | |||
|
Follow-up |
Gained >2 lines |
No change in acuity |
Lost >2 lines |
|
3 months |
49% |
34% |
17% |
|
6 months |
48% |
44% |
11% |
|
Table 2. Anatomical Results of Kenalog for Vein Occlusion | ||||
| Follow-up | Gained >2 lines |
No change in acuity |
Lost >2 lines |
Edema worsened |
|
3 months |
49% |
34% |
17% |
28% |
|
6 months |
48% |
44% |
11% |
27% |
Retinal physicians from Texas and researchers from Alcon are reporting 12-month results of two open-label studies of anecortave acetate (Retaane, Alcon) administered as a posterior juxtascleral depot in patients with minimally classic, occult and/or predominantly classic CNV lesions due to AMD.
Study 1 used a 30-mg dose, while Study 2 used 15 mg. Twenty-two of 34 patients enrolled in Study 1 and 10 of 20 patients in Study 2 received concomitant PDT with their anecortave. Seventy-two percent of the 30-mg group and 81 percent of the 15-mg group maintained their vision after a year of treatment, which included two PJD administrations. All lesion types performed equally well. Though there was no systemic accumulation of anecortave desacetate or other metabolites after repeated administration of anecortave acetate, lower plasma concentrations were seen in patients with reflux of the agent.1375
VEGF Inhibition and AMD
Researchers in the VEGF Inhibition in Ocular Neovascularization (VISION) Clinical Trial Group say that pegaptanib sodium injection (Macugen, Eyetech/Pfizer) remains effective in CNV eyes two years after treatment, and that it's safety profile remains favorable.
Patients with all angiographic subtypes of AMD were included in two randomized, double-masked, controlled, multicenter studies. During the first year, patients received either intravitreal pegaptanib injection (0.3 mg, 1 mg, 3 mg) or sham injection every six weeks for 54 weeks.
Patients originally assigned to pegaptanib were then re-randomized (1:1 ratio) to continue or discontinue therapy for an additional 48 weeks (eight injections). Those assigned to sham in the first year were re-randomized either to continue sham, discontinue it or receive one of the three pegaptanib doses.
At week 102, the researchers assessed 941 re-randomized patients who had received an average of 15.7 of a possible 17 injections over two years. The patients who received a 0.3-mg dose of pegaptanib for a second year lost an average of 9.4 letters of acuity, compared with a loss of 17 letters for controls. Fifty-nine percent of the re-randomized pegaptanib patients lost fewer than 15 letters, compared to 45 percent of controls. There were 67 percent more events of three-line vision loss in those receiving just one year of treatment compared to those receiving two years. No new ocular or systemic safety issues emerged during the second year.2309
Researchers from the Bascom Palmer Eye Institute and the University Eye Hospital in Vienna are conducting an open-label, uncontrolled study of Avastin (bevacizumab, Genentech), a drug currently only approved in the United States for the treatment of patients with colorectal cancer. The researchers initiated the study in order to find out if Avastin could improve acuity outcomes in wet AMD through its anti-vascular endothelial growth factor (VEGF) properties. They are also presenting an abstract of bevacizumab's safety profile at this year's meeting.
To qualify for the study, patients had to have subfoveal CNV, a central retinal thickness of at least 300 µm and acuity between 20/40 and 20/400. They were treated initially with two or three infusions of 5 mg/kg of bevacizumab at two-week intervals.
Of the 15 patients in the study, the first nine have been followed for at least three months, and they've received two or three infusions of drug so far. By three months, the median and mean visual acuity letter scores of these nine patients increased by eight letters and 12 letters, respectively. In the nine fellow eyes, eight were diagnosed with CNV at the baseline visit. In these fellows, median and mean acuity increased by 27 letters and 16 letters, respectively.
At six weeks, the only significant systemic side-effect was a mild elevation of 12 mmHg in the median and mean systolic blood pressure measurements, which was controlled by changing or initiating anti-hypertensive medication. By 12 weeks, the elevation of systolic blood pressure was no longer significant. There were no other serious adverse events. Of course, these results are over a short term, and the researchers note that they will continue to follow the patients to determine the durability of these favorable visual outcomes.2310,1378
Researchers in the Genentech-sponsored trial of intravitreal ranibizumab (Lucentis) for subfoveal CNV secondary to AMD are reporting the latest long-term results of the treatment.
Patients who lost fewer than 15 letters of vision at the conclusion of the Phase I/II studies were able to continue on ranibizumab in their study eyes in an open-label extension study. Of 70 subjects enrolled, 66 received at least one ranibizumab injection. By amendment to the original protocol, the researchers increased the initial 0.3-mg dose to 0.5 mg, and the fixed dosing interval of every four weeks was changed to a more flexible strategy of holding a dose if the acuity was stable (defined as a change of fewer than five letters) and lesion characteristics were stable on two consecutive visits.
The researchers have analyzed safety and efficacy results up to April of last year. The median follow-up was 415 days (range: 70-589 days). The median rate of dosing was 0.22 injections every four weeks, a reduction from the pre-amendment rate of one injection every four weeks. Visual acuity showed no overall decline during the study, and ranibizumab was well-tolerated. The most common adverse events were:
• conjunctival hemorrhage (29 percent);
• eye pain (11 percent);
• blurred vision (11 percent);
• iris and uveal-tract inflammations (11 percent); and
• increased IOP (11 percent).
All but one of these events were of mild or moderate severity. The most common non-ocular adverse events were nasopharyngitis (12 percent) and mild increased blood pressure (14 percent).1393
Doctors from the Fluocinolone Acetonide Implant Study Group will share the one- and two-year results of their study of Bausch & Lomb's sustained-release fluocinolone implant for patients with diabetic macular edema.
In the study, they randomized 197 patients to receive either a 0.59-mg implant or standard of care (repeat laser or observation) in a 2:1 ratio.
At one year, 57 percent of eyes receiving an implant had no evidence of edema at the center of the macula, compared to 20 percent of the control eyes. Also, 56 percent of the implant eyes had a two-step improvement in retinal thickness at the center of the macula compared to just 17 percent of the control group. Seventeen percent of the fluocinolone eyes showed a greater-than-one step decrease in diabetic retinopathy severity scores vs. 5 percent of control eyes. However, at a year, there was no difference in visual acuity between the groups.
The most common adverse events in the study group were serious cataract progression (43 percent) and serious IOP rise (8.6 percent); five eyes (3.9 percent) required filtering procedures. The researchers say they will present the extended two-year follow-up results at ARVO.4673
In a Phase-II study supported by Eyetech and Pfizer, researchers say that Macugen was both well-tolerated and effective for the treatment of diabetic macular edema.
 |
| A controlled study found the 0.3-mg dose of Macugen was effective for treating diabetic macular edema. |
The 0.3-mg dose group proved to be the best performer, and at nine months its average acuity was better than sham. The visual acuity results appear below:
|
Table 3. Pegaptanib for Diabetic Macular Edema | ||||
|
Dosage |
Gained >15 letters |
Gained >10 letters |
Gained >5 letters |
No Change/improved |
|
0.3mg |
18% |
34% |
59% |
73% |
|
Sham |
7% |
10% |
34% |
51% |
Surgical Insights
Turkish researchers say surgeons should consider using adjuvant 5-fluorouracil and low-molecular weight heparin in pars plana vitrectomy for complicated cases of rhegmatogenous retinal detachment repair.
The study was prospective, randomized, double-blinded and placebo-controlled. The two randomized groups were those in which 5-FU and heparin were added to the vitrectomy infusate and a group in which only BSS was added.
The first group (n=41) consisted of 15 eyes with post-traumatic retinal detachment, 21 eyes with severe proliferative vitreoretinopathy, and five with giant retinal tears and severe PVR. The placebo group (n=41) included 14 eyes with post-traumatic retinal detachment, 24 with severe PVR and three eyes with giant retinal tears. All eyes had total retinal detachment with macular involvement. The preop median PVR grade was C3 in both groups, and preop median best-corrected acuity was hand motion.
With an average follow-up of nine months, 24 percent (10 eyes) of the treatment group and 46 percent (19 eyes) of the control group underwent reoperation for recurrent retinal detachment due to PVR. Redetachment rate was statistically significantly higher in the control group, but there was no significant difference between the groups in terms of postop complications such as fibrin, hemorrhage, IOP elevation or corneal edema.3626
Researchers from the multicenter TTT4CNV study group say that transpupillary thermotherapy hasn't shown a benefit vs. sham treatments overall, but has shown a benefit in eyes with poor baseline acuity.
The researchers enrolled 303 patients with subfoveal occult CNV with a maximum lesion diameter of 3 mm and acuities ranging from 20/50 to 20/400. The patients were randomized in a 2:1 ratio of TTT to sham, and retreatment was allowed only at three months.
The investigators say that an intent-to-treat evaluation of the primary visual outcome data show that TTT didn't result in a significantly beneficial effect relative to sham. They then evaluated the results in eyes with acuities of 20/100 or worse (n=124). At months 12, 18 and 24, the average loss of vision in these patients was less in the TTT group by five to nine letters. Improvement of acuity by two or more lines was significantly greater in TTT-treated eyes than sham at one year (19 percent vs. 0) and 18 months (17 percent vs. 0). The best results occurred in TTT eyes that weren't retreated.2311
Dr. Regillo is professor of ophthalmology at Thomas Jefferson University and director of the Clinical Retina Research Unit at Wills Eye Hospital.
*Reference numbers noted as footnotes correspond to the abstract numbers in the 2005 ARVO program.