The Mechanics
The MEL-80 is an excimer that operates at a repetition rate of 250 Hz, using a 0.7-mm flying spot. It is approved to treat up to -7 D with up to -3 D of cylinder. If someone has the maximum amount of cylinder, however, the maximum sphere that can be corrected is -5.5 D.
"The first thing that's different about it is it's significantly faster than some lasers," says Mark Packer, MD, who participated in the MEL-80's FDA trial. "Since it's a 250-Hz laser, the ablation time for a -4 D eye is 15 seconds, so it's very fast. There's not a lot of time spent watching the ablation. Now, this speed doesn't mean a great deal in terms of office efficiency, but it does mean increased predictability and accuracy of the ablation, because there's less time for the cornea to hydrate while the flap is up and the treatment is ongoing."
Jon Dishler, MD, of Greenwood Village, Colo., has used the laser in its hyperopia trial, and appreciates one of its small features. "Some lasers need a tank of nitrogen to allow the gas to flow through the laser, purging the optics of room air so that they'll work properly," he explains. "However, there are some issues with that approach. The MEL-80 is unique in that it makes the whole laser path into one big vacuum tube that provides a stable environment that causes very little energy to be lost from the beam as it passes through the optics. This produces a very stable, reproducible laser output." Dr. Dishler also says the calibration process of the laser is very simple, with no plastic samples to ablate and analyze. "The laser has a simple calibration film that you shoot and then get a straightforward 'go/no-go' kind of report."
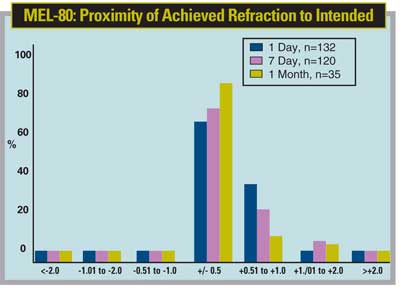
The MEL-80 uses an aspheric ablation profile in an effort to preserve the eye's natural shape postop. The designers hope that this decreases the induction of spherical aberration and qualitative vision problems postoperatively.
"The ideal profile can maintain the eye's asphericity and not interfere with visual quality," says Antwerp, Belgium's Frank Goes, MD, whose MEL-80 data was used as part of Carl Zeiss Meditec's FDA application for approval. "For example, with the older laser ablation profiles, some patients had problems driving at night or had induced spherical aberration. This profile avoids that. It takes more tissue though, the amount of which you'll have to calculate beforehand to determine if the procedure will be safe or not. A helpful part of this profile is that we can keep the ablation zone small. With the MEL-80, 6 mm is the standard-size zone, even for large pupils, so it's rare that you have to use a larger one, meaning you can take less tissue with the aspheric profile." Dr. Goes says the extra amount of tissue taken in comparison to a non-aspheric treatment varies, and depends on the astigmatism to be corrected.
"If there is a lot of astigmatism, the extra tissue can sometimes be 35 to 40 percent," says Dr. Goes.
Dr. Packer says this increased removal of tissue is the "other side of the coin" when using prolate optimization software. "You reduce the applicable range of the laser because you're removing a bit more tissue," he says. "That's part of the reason that the approval was limited to -7 D, even though we were going up to -12 D in the trial. There were few eyes that had enough tissue to allow ablation beyond -7 D. So, even with a -8 or -9 D patient, whom you might be able to treat with another laser and not run up against the 250-µm residual bed tissue limit, with this laser, you do."
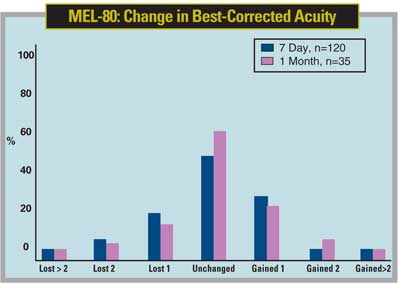
Dr. Dishler says that, though the laser is accurate and effective—with 40 percent of patients in the trial seeing 20/12.5 or better—the initial approval of up to -7 D may mean it's not currently a "single-source solution." He adds, though, that the approval range accounts for probably 85 percent of the patients a refractive surgeon is likely to see.
The system also has an eye tracker that tracks the pupil and limbus.
"The MEL-80 uses a video eye tracker that captures the image from three different directions," says Dr. Packer. "It also operates at 250 Hz, so it doesn't lag behind the laser pulses. To use the tracker, you swing it in before you lift the flap, and it captures the image then. This differs from some lasers that require the flap to be lifted first, which may lead to increased corneal dehydration ."
Dr. Dishler says you can also modify what the tracker sees as the center. "If you want to have a slight offset, such as in a hyperope where you'd want to track at the apex of the cornea, it's easy to adjust the tracker slightly nasally to track that point instead of the center of the pupil," he says.
Though the MEL-80 uses sophisticated aspheric ablation algorithms and boasts excellent clinical trial visual acuity results such as the 41 percent of patients seeing 20/12.5, surgeons may wonder if they should go with a custom-guided laser. The surgeons we interviewed understand how their colleagues could be in a quandary over this, but they think the MEL-80 will treat a majority of a practice's patients very well.
Dr. Goes thinks the laser would be usable in 80 to 90 percent of patients. "I don't use wavefront-guided ablations for standard treatments," he says. "I use them for certain conditions, such as enhancements or retreatments where there are a lot of aberrations. The prolate, optimized ablation profile will suffice for many refractive patients."