Surgery always comes with some amount of risk, and removing a cataract is no exception. Postoperative inflammation—and more concerning, endophthalmitis—are potential consequences that cataract surgeons go to great lengths to prevent. Today, with technology advancing rapidly, new options for preventing these undesirable outcomes are appearing. Nevertheless, many surgeons are hesitant to abandon current protocols, and some surgeons say that many effective low-tech precautions are still frequently overlooked.
Here, surgeons share their experience and advice regarding how to do the best possible job of preventing postoperative inflammation and endophthalmitis—whether or not you’ve adopted any of the cutting-edge protocols.
To Drop or Not to Drop?
The biggest debate right now centers around how to manage the patient at the end of surgery and into the postoperative period. Although giving the patient a battery of preop and postop
eye drops has been the standard approach for a number of years, there are two clear arguments for switching to an injection of antibiotic (and possibly other drugs) at the conclusion of surgery: First, evidence is mounting that injecting antibiotics is effective at preventing postop infection—possible more effective than having the patient use drops. Second, drops are a huge burden for the patient and the practice, so the fewer drops the patient has to use, the better.
“Don’t expect to reduce your risk of endophthalmitis by having the patient use antibiotic drops for a week before, or two weeks after, surgery,” says Robert M. Kershner, MD, MS, FACS, professor and chairman of the Department of Ophthalmic Medical Technology at Palm Beach State College, and president and CEO of Eye Laser Consulting in Palm Beach Gardens, Florida. “There’s not a single study showing that this has any substantive effect. The only solid evidence that shows antibiotics to be of any benefit is when they get inside the eye at the time of surgery. In addition, there’s a moderate amount of evidence that suggests that using postoperative antibiotic drops in addition to an intraoperative intracameral or intravitreal injection may lower the incidence of endophthalmitis, compared to using injections or eye drops alone. So it’s probably best to use a combination.”
“We’re at the forefront of a huge paradigm shift in postoperative drug delivery,” says Cynthia Matossian, MD, FACS, founder and chief medical officer of Matossian Eye Associates, and a clinical assistant professor of ophthalmology (adjunct) at Temple University School of Medicine in Philadelphia. “By eliminating drops, we’re going to take compliance out of the patient’s hands. We’ll eliminate the confusion and difficulty of managing the drops, and decrease the burden for the patient and the patient’s family. In addition, we’ll be putting the drugs closer to the target tissue.
| Making the Preop Period Count |
| It’s fairly common for cataract surgeons to put their patients on drops prior to surgery, but many surgeons are questioning the value of that protocol. Nick Mamalis, MD, a professor of ophthalmology and director of ocular pathology at the University of Utah in Salt Lake City, says he hasn’t found the use of antibiotic drops prior to surgery to make a significant difference in the incidence of postop endophthalmitis. “We’ve had issues getting patients to be compliant with the use of drops preoperatively, so we don’t start anything until the patient arrives at the surgery center,” he says. “After they arrive, patients get three sets of a fourth-generation fluoroquinolone, like moxifloxacin or gatifloxacin, several minutes apart, while they’re receiving dilating drops and topical NSAIDs,” he says. “We include the NSAID because there’s some evidence that having an NSAID onboard before making your incisions will decrease the breakdown of the blood-aqueous barrier in the anterior chamber during cataract surgery. In fact, if the patient has an increased risk of inflammation after surgery—for example, if the patient has a history of uveitis or inflammation in the eye—we’ll often start the patient on NSAID treatment up to a week prior to surgery to reduce the chance of a breakdown of the blood-aqueous barrier.” Dr. Mamalis believes that preoperative povidone iodine is much more effective at ensuring sterility than preoperative antibiotics. “There’s very good evidence that 5% povidone iodine is helpful,” he says. “So, when patients are in the preop holding area we give them a drop of topical povidone iodine. However, it’s important to do this before giving the patient any lidocaine gel to anesthetize the surface of the eye. If you put the gel in first, it will prevent the povidone iodine from spreading out and covering the surface of the eye as it should. Once the patient is in the OR undergoing prep for surgery, the skin is prepped with 10% betadine, but we also put another drop of the 5% betadine on the surface of the eye. By this point the gel has been in the eye for 10 or 15 minutes and has dissipated a bit, so it doesn’t interfere with the coverage of the iodine.” —CK |
“The average cataract patient in the United States is in his or her late 60s or early 70s,” she notes. “Managing a complicated schedule of drops can be very challenging for some patients. For example, a steroid drop is tapered over a period of several weeks; an antibiotic drop is stopped earlier than the steroid and the NSAID. A brand-name NSAID is used once a day, but if the pharmacist substitutes a generic drop, it may be used four times a day. This creates a lot of confusion—and this is just the first eye. After few weeks we do eye two, which has a different schedule that’s not aligned with whatever remains for eye one. To complicate matters, some patients are forgetful. They may be confused; they may have arthritis in their hands and not be able to squeeze the bottle appropriately; they may contaminate the tip; and they may think they put the drop in when it actually ended up on their forehead or eyelid.
“In addition, the cost of the drops is becoming prohibitive,” she continues. “The cost can be as much as $400 or $500. With higher deductibles and a greater percentage of responsibility being placed on the patient by insurance carriers, patients are experiencing sticker shock when they go to pick up their drops. And when they have sticker shock, guess what they do? They call our offices and yell at our staff. They believe we’re the cause of the high prices, even though we have nothing to do with it. Furthermore, when the pharmacist substitutes generic drops, the frequency protocol changes; that confuses the patients even more, so they call us to get clarification. Aside from having unhappy patients, the number of incoming calls about these issues is becoming burdensome. Dealing with upset patients, tying up our phone lines and technician time, is a cost to the practice.
“For all of these reasons,” she concludes, “minimizing the drop-instillation regimen is the way to go.”
Going Intracameral
“There’s good evidence from multiple studies around the world that intracameral antibiotics are effective at preventing endophthalmitis,” says Nick Mamalis, MD, a professor of ophthalmology and director of ocular pathology at the University of Utah in Salt Lake City. “So, we put one-tenth of a cc of preservative-free 0.5% moxifloxacin into the anterior chamber at the conclusion of the case. Also, after we take the speculum out and peel the covers off, I like to put an additional drop of a fourth-generation fluoroquinolone on the eye, along with another drop of betadine, before we put the shield on.”
It’s clear that despite mounting evidence that intracameral injection of antibiotics is effective, many American surgeons are hesitant to switch to this protocol. Dr. Mamalis points out that one reason many surgeons haven’t switched is that there’s no drug that’s FDA-approved for this purpose. “Surgeons would prefer to have an approved postoperative antibiotic that comes in a single-use vial, specifically made for injection following cataract surgery for prevention of endophthalmitis,” he says. “A lot of people have said that without this, they’re concerned about the formulation and how you get it made. I think that’s keeping many surgeons from switching. I’m lucky to be at a university, because we have our own pharmacy. They’ll put a preservative-free antibiotic into a syringe for us under sterile conditions, so we can just inject it directly. Without that advantage, surgeons have to find an outside source on their own.”
Even some surgeons who have switched to intracameral antibiotics are still using postoperative drops as a safety measure. Dr. Mamalis admits he hasn’t committed to eliminating postoperative antibiotic drops yet, despite his use of an intracameral antibiotic. “I’m not sure if it’s because we’re creatures of habit, or simply still worried about postoperative endophthalmitis, but we use a belt-and-suspenders approach,” he says. “We use the intracameral antibiotic but also have the patient use a topical fourth-generation fluoroquinolone for a week postoperatively. I think this is reasonable, because we don’t know for sure whether there might be a problem several days after the surgery. I prefer to err on the side of caution.”
Sheri Rowen, MD, who practices at NVision Eye Centers in Newport Beach, California, and is a clinical assistant professor of ophthalmology at the University of Maryland, notes that even though there is telling evidence throughout the world supporting the postop injection of antibiotics, she hasn’t seen any long-term studies confirming the efficacy of this approach.
“We’ve seen some debate about which antibiotic can be used, and some cases of hemorrhagic occlusive retinal vasculitis (HORV) have occurred,” she points out. “Many surgeons using this protocol are injecting non-preserved moxifloxacin, but a lot of people are trying other alternatives internationally. When we’re at American meetings and we raise hands to see who’s using this approach, it’s not a compelling majority of cataract surgeons yet—at least in the U.S.”
Dr. Mamalis notes that the American Society of Cataract and Refractive Surgery has put together a research council to conduct a series of research studies that could potentially lead to FDA approval of an intracameral antibiotic. “A study like this could only be done through a large group with a large number of surgeons involved,” he says.
“The first project we’re tackling is a masked study comparing an intracameral injection of a fourth-generation fluoroquinolone to topical drops for prevention of endophthalmitis,” he explains. “Because endophthalmitis is rare, we’ll need to enlist as many as 75,000 patients. But if this study proves that an injection of intracameral antibiotic is efficacious, it will allow companies to get approval to make a preservative-free antibiotic for single use after cataract surgery. This will go a long way toward making surgeons more willing to use an intracameral antibiotic.”
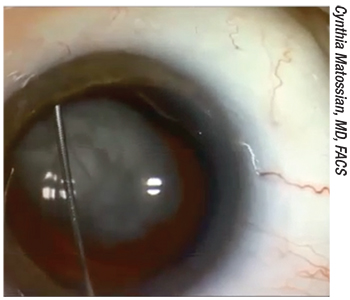 |
| One approach to placing drugs inside the eye has been to place a bolus of drug into the vitreous, usually by inserting a cannula through the zonules (as shown above). Given the potential for complications—and patients often dealing with floaters for a period of time afterwards—many surgeons have been unwilling to adopt this approach. |
Can We Go Dropless?
Dr. Matossian says she’s been doing everything possible to avoid giving the patient postoperative drops for the past two years. “At the conclusion of cataract surgery, just before I seal the incision, I put a steroid and antibiotic combination into the anterior chamber with a 25-ga. bent cannula,” she explains. “I use Dex-Moxi, which is dexamethasone and moxifloxacin; it’s clear, like water. Once it’s placed inside the eye, I seal the incision in the normal way.” Dr. Matossian notes that the downside to her current approach is that neither version of Dex-Moxi (one from Imprimis, one from Ocular Sciences) is FDA-approved. “The combination drugs are manufactured by a 503-A or -B pharmacy,” she explains. “We order it per patient, and the formulation is sent to the ASC.”
Dr. Matossian adds that she still has patients use an NSAID once a day postoperatively. “That’s far easier for the patient than the multi-drop protocol,” she points out. “The exception is if I’ve done a limbal relaxing incision. In that situation the patient also needs topical antibiotics, which I prescribe b.i.d. for five days. In addition, if it was a complex case in which I went in and out of the eye more than normal, or I used a pupil dilating device or the case was prolonged, I might add a topical steroid for just a few days in addition to what I’ve placed intracamerally.”
Dr. Mamalis acknowledges that few or no postoperative drops would be good for both patients and practices. “The problem is that there’s not enough data out there for us to really know how well this works,” he says. “In addition, there’s no commercially available approved medication to inject for this purpose. That means you have to go through a compounding pharmacy to get the medication made up. Furthermore, you can’t just put it in the anterior chamber; you have to leave a depot of this medication in the eye, either placing it through the zonules or in the anterior vitreous.”
Dr. Rowen says she thinks the jury is still out on how surgeons will use intraocular antibiotics. “Everybody’s trying to figure out exactly what they’re comfortable with and what they feel they need,” she says. “If you haven’t had a case of endophthalmitis in 20 years, you might feel like you’re doing OK with your current protocol. I think if anything new will be adopted, it will be an intracameral injection done at the end of the case—but I can’t tell you what the majority of doctors will do. There’s no consensus yet. We all want to be in line with the accepted standard of care, but I don’t know that we have an accepted standard of care in this area.”
Stop Infection Before It Starts
Dr. Kershner emphasizes something that’s often omitted from the discussion: If a postop infection occurs, it started during the surgery. In fact, he points out that the term “postoperative endophthalmitis” is misleading. He says the best way to prevent endophthalmitis is to do everything humanly possible to ensure that sterility is maintained during the procedure, rather than focusing on postoperative fallbacks.
“We’re probably infecting many more patients than we realize,” he says. “Fortunately, in most cases the end result isn’t endophthalmitis. The result may be inflammation that we interpret as a low-grade sterile inflammation, when in fact the postoperative cell and flare is secondary to intraocular organisms. Even if that happens, in most patients with a normal immune system—though not all—receiving intraoperative antibiotics will likely ensure that a contamination doesn’t turn into an inadvertent adverse event.
“The point is that what goes on during the surgery is a lot more important than what happens before or after the surgery,” he says. “Preoperative and postoperative antibiotic drops or injections can minimize the chances of endophthalmitis, but preventing it during surgery makes the most sense. That will always be your best insurance policy.”
Dr. Kershner notes that some ophthalmologists might be lulled into the illusion that contamination is unavoidable, and if an infection occurs an antibiotic will take care of the problem. “Let me disabuse them of that notion,” says Dr. Kershner. “Many times the organism is not identified right away, and a frightening number of bugs are now resistant to most of the antibiotics we’ve used in the past. Surgeons must keep in mind that no amount of antibiotic is going to be a substitute for strict adherence to preoperative and intraoperative attention to sterile technique. All ophthalmologists need to take this into account when deciding what they’re going to do to prevent endophthalmitis.
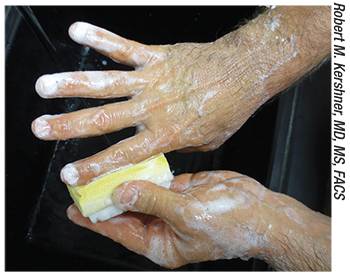 |
| The best way to prevent endopthalmitis is to make sure sterile protocols are followed to the letter during surgery, including a thorough preoperative scrub. |
“In the past, when a patient developed an infection, a team of infectious disease experts would be brought in to examine everything associated with that surgery to determine where the bacteria came from,” he recalls. “With good sleuthing, we usually could identify the source. Normally, the ocular surface is already colonized with a variety of resident surface flora, including the common bacteria Propionibacterium acnes followed by coagulase-negative Staphylococcus, Corynebacterium, Streptococcus sp., Enterococcus sp., and to a lesser extent gram-negative bacteria and fungi. In one case I was made aware of, an eye had become infected with the intestinal microflora Morganella morganii, which is not a common organism to find inside the eye, to say the least. How could an organism get from below the waist into the eye? The answer: poor hygiene. Not on the part of the patient, but a member of the surgical team.
“The infectious disease team came in and swabbed everything, including all body parts of the patient and everyone who was in the OR,” he continues. “In this instance, the patient didn’t carry the organism, but one technician did. Apparently, after multiple cases, the tech went to relieve herself and was not scrupulous enough about thorough washing of her hands, and rescrubbing as if it were the first case of the day.”
Ensuring Sterility
Dr. Kershner points out that there are a number of steps every practice should take to minimize the chance of contamination during surgery. First, policies designed for this purpose should be stated and enforced.
• Make sure your techs understand that they have to be scrupulous about sterility. Dr. Kershner notes that when the patient load is heavy, people may drop their guard. “In many surgical centers, they’re turning over those rooms pretty fast, and it’s easy to skip a step in the preoperative scrub routine, such as rushing the scrub or using an antiseptic lotion as a substitute for a thorough hand scrub done with antiseptic soap and a brush,” he says. “Of course, the one time you don’t do a proper scrub is the one time that you’re going to contaminate the patient and end up with an infection.”
• Create a written protocol describing exactly how every eye should be prepped. “You should have a written protocol specifying how to properly prep an eye for surgery,” says Dr. Kershner. “I’ve seen all kinds of poor protocol. I’ve seen the OR crew put in a couple of drops of betadine followed by a half-hearted wipe of the eyelids with a cotton swab, and then decide that’s good enough. I’ve seen doctors fail to exclude the eyelashes and eyelid margin from the operative field and then drag instruments over them. These types of mistakes will eventually get you into trouble.
“In fact, if you want to go after the resident flora, or more importantly, the transient microbial flora, you have to be meticulous about the preoperative preparation of the patient—what touches the eye, what gets into the eye, what gets out of the eye, and how and what is used to assure strict antisepsis,” he continues. “Antisepsis doesn’t work unless the antiseptic agent reaches every surface of the skin, eyelashes, and bulbar, palpebral, perilimbal and forniceal conjunctiva, and is left on those surfaces for a significant amount of time.”
• Take the time to train your techs to follow sterile procedure, yourself. “I don’t leave technician training to someone else,” says Dr. Kershner. “Maybe they received good training before working with me, maybe they didn’t. But the surgeon is the one who has to make sure everything is done correctly. So, I show my ophthalmic medical technologists, scrubs and OR nurses exactly how I expect them to scrub before surgery.”
• Set an example. Dr. Kershner says that he always makes sure to follow his protocol to the letter. “I make sure that I’m a good example—that I always scrub properly and for long enough to make a real difference,” he says. “I never scrub halfheartedly and throw on some sanitizing gel as a substitute for a thorough scrub.”
• Make it clear that being the cause of contamination is grounds for dismissal. “I explain that if anyone doesn’t follow this routine each and every time, they can look for a job elsewhere,” Dr. Kershner says. “I tell them, ‘If we get a case of endophthalmitis, we’re going to go looking for the culprit, and if it’s you, you’ll lose your job.’ ”
| The Transzonular Approach |
| One approach to placing a drug, or drugs, inside the eye has been to place a bolus of drugs into the vitreous, usually by inserting a cannula through the zonules. Cynthia Like many surgeons, Sheri Rowen, MD, who practices at NVision Eye Centers in Newport Beach, California, says she was never comfortable with the idea of placing a bolus of triamcinolone and antibiotic into the eye through the zonules. “Some people found that approach to be within their comfort level, but a lot of us didn’t,” she notes. “One reason I never used that protocol is that it can cause floaters; the patient has to see through the triamcinolone for a while. Retinal detachments have also been reported. I wasn’t comfortable with that, nor did I feel I needed that in order to give the patient a good outcome. In addition, some doctors have had problems with some compounding pharmacies.” “Now we have two FDA-approved products [available soon] that can deliver a steroid in the post-cataract period,” Dr. Matossian adds. “I’d rather use one of these two options than a non-FDA-approved product—and they won’t require placing anything into the vitreous.” —CK |
Preoperative Pearls
Once the basic rules regarding sterility have been established, these strategies will help ensure that no unnecessary sources of contamination make their way into the OR:
• Examine the patient preoperatively. “I’ve always believed that the surgeon should see the patient before the surgery—and not just after the patient has been prepped and is in the OR,” says Dr. Kershner. “Sometimes the surgeon hasn’t seen the patient for weeks. I’ve had patients say they wanted to finish the golf tournament, or go up north and visit the kids and think about surgery; then they call two months later and say, ‘OK, let’s do it.’ If that happens, my staff will tell the patient we have to see him again to re-examine and go over everything. The patient may have developed a cutaneous inflammation or infection—acne rosacea, seborrhea or blepharitis. Perhaps the signs of these were missed during the last examination, but those conditions need to be addressed before contemplating surgery. The patient may now be on antibiotics for some reason, or have recently had dental surgery, or have a pulmonary or urinary infection. It can get pretty expensive and time-consuming to discover this kind of thing at the last minute when the patient is in the holding area ready for surgery—not to mention the patient’s disappointment. Or worse, we may put the patient at risk by not discovering it at all.”
• Look carefully at the patient’s chart. “As the surgeon, you need to stop before and between cases, take a breath, and look carefully at the history of the patient you’re about to operate on before commencing surgery,” notes Dr. Kershner. “I’ve seen cases in which concerns regarding symptoms and signs were noted in the chart, but the surgeon who reviewed the chart missed them. I’ve reviewed other cases where the surgeon did see the patient prior to surgery, but zoomed in and out without doing a careful exam,” he says. “As a result, the surgeon missed something that could have put the patient at risk. A little time spent in advance of the surgery could make all the difference between an uneventful surgical case and a courtroom.”
• Check for infections elsewhere in the body. “Doctors often don’t ask about or check to see if the patient has an infection elsewhere in the body, such as a urinary tract infection,” notes Dr. Kershner. “These individuals have a certain degree of bacteremia; they’re already contaminated. Infections elsewhere can end up becoming systemic infections, and those organisms sometimes can show up in the eye. If these factors are not reviewed by the surgeon, and noted in the patient’s record, then they can be missed.”
• Train your staff to note potential problems and bring them to your attention. “I always remind my personnel that if anything is discovered—even at the last minute—I want to hear about it,” he says. “Unfortunately, taking the history is usually relegated to the lowest person on the totem pole, so the person who does ask the right questions may not want to bother the doctor with the information—information that may be critical to the patient’s well-being. Tell your staff, ‘If the patient has a systemic infection, let me know. If the patient just had a boil on his neck lanced, point that out to me.’ ”
• Talk to the patient prior to surgery. Dr. Kershner advises: “Ask the patient: ‘How are you feeling? Have you been sick recently? Have you had any procedures performed, such as dermatological, dental or urinary tract procedures? Have you had a colonoscopy? Are you on any medications that you weren’t on the last time I saw you?’ This is part of our job as doctors. It’s the surgeon who doesn’t take the time to do this before surgery who gets in trouble after surgery.”
| “It’s critical that your injection be preservative-free and the proper dose.” —Nick Mamalis, MD |
Dr. Kershner adds that you shouldn’t wear a mask when talking to the patient. “Let the patient see that it’s you, and make it clear to the patient by your demeanor and the questions you ask that you’re concerned about his or her welfare,” he says. “Finally, don’t wait until the patient is prepped and in the OR to have this conversation. If that’s the norm for you, think about changing your approach. Talking to the patient in the OR is too late.”
During the Surgery
These intraoperative strategies will help reduce any chance of infection:
• Minimize your instrumentation. “There’s usually no need to put instruments in and out of the eye multiple times during cataract surgery,” notes Dr. Kershner. “Every time you do that you increase the risk that bacteria will get inside the eye.
“In general, you should always use the minimum number of instruments you can use,” he continues. “This has multiple advantages. First, fewer instruments means less sterilizing and managing of tools before, during and after the procedure. Second, the less often you introduce foreign objects into the eye, the safer the procedure becomes. Third, it’s efficient, meaning it takes less time to complete your surgical task and the patient spends less time under the microscope. That’s important because the less time the procedure takes to complete, the lower the risk that something bad will happen while you’re working in the eye.
“An efficient and well-executed procedure also translates into faster recovery and a better outcome for the patient,” he continues. “Show me a doctor who uses 15 instruments and takes 20 minutes to do the surgery, and I’ll show you a patient with corneal and macular edema and weeks before they achieve clear vision. Of course, being efficient doesn’t mean you have to rush. Hurrying is never in the patient’s best interest.
“If you need a special instrument,” he adds, “the tech can easily grab a sterile, sealed blister pack with the additional tool.”
• Know exactly what you’re injecting. “It’s critical that your injection be preservative-free, and the proper dose,” says Dr. Mamalis. “If you’re going to inject moxifloxacin, some studies have shown that one-tenth of a cc of preservative-free 0.5% moxifloxacin is safe and nontoxic.”
• Inject through the side-port incision. “First of all, make sure that your primary wound is watertight,” says Dr. Mamalis. “Then, at the conclusion of the case, you can make the injection through the side-port incision into the anterior chamber. Injecting through the small, less-than-1-mm side-port incision avoids disturbing the main incision.”
• Make sure your wound is absolutely watertight. “A leaky wound is the most serious risk factor for postoperative endophthalmitis,” notes Dr. Mamalis. “Studies, including some done at our institution, have found that a wound leak increases the risk of endophthalmitis 44 times. Not 44 percent, but 44 times! For that reason, we’re very compulsive about checking this before we leave the OR.
“To ensure that the wounds are watertight, we hydrate the wound and stab incisions,” he explains. “Then we bring the anterior chamber up to physiological pressure, dry the incisions with a Weck-Cel sponge, and put gentle pressure on the eye at the limbus on the opposite side. Under high-power microscopy we make sure there’s absolutely no leak. If there’s any question, you can put a little fluorescein on there and perform a Seidel test. In the uncommon situation that we can’t get the wound to seal, we’ll put in a suture.”
Two Final Thoughts
Last but not least, these two strategies will help make sure that you’re giving your patients the greatest chance of an excellent outcome:
• Accept responsibility for any less-than-ideal outcomes. Dr. Kershner says this was ingrained in him when he trained as a general surgeon before going into ophthalmology. “We were taught that the surgeon is responsible for everything that happens to the patient,” he says. “If you had an infection postoperatively, it was your fault and most likely secondary to poor surgical technique. The surgeon simply did not blame the patient. Assuming responsibility for any problems that might occur following surgery will ensure that you go the extra mile to prevent every type of contamination that’s within your ability to control.”
• When new options appear, try them—and share your experience. “The only way we can really know what works best in our hands is to try it on our patients,” says Dr. Rowen. “We can read how well something worked in an FDA study, but that doesn’t necessarily reflect how well it will work in the real world. I prefer to stick with products that are approved, but once they’re approved, I think it’s important to try them and share what we learn. That’s how we decide what persists in our field.”
Dr. Matossian notes two new products that are FDA-approved that should be available shortly. “One is called Dexycu [Eyepoint]; it’s a 9% dexamethasone intracameral ophthalmic steroid suspension that’s placed under the iris using a 25-ga. bent cannula at the conclusion of cataract surgery,” she explains. “Through its ‘Verisome’ technology, it delivers more steroid at the beginning; then over a 30-day period, it bio-erodes and disappears. Thus, it mirrors our steroid tapering schedule.” This may allow surgeons to eliminate the steroid drop with an FDA-approved alternative.
“The second approved product that should be available soon is called Dextenza,” Dr. Matossian says. “It’s an intracanalicular insert that contains dexamethasone 0.4%; it’s placed into the inferior punctum like a punctal plug at the conclusion of cataract surgery. It elutes dexamethasone for 30 days to treat postoperative pain. It’s another option that will allow us to eliminate postoperative steroid drops. Of coure, there will be some situations in which the punctal anatomy may not be suitable for inserting the Dextenza plug. I think that with experience, we’ll know which product is better suited in specific circumstances.”
She adds that the possibility of skipping an NSAID drop is also on the horizon. “There’s a punctal plug in the pipeline that contains an NSAID that elutes over time, but that’s not available yet; it’s still in clinical trials,” she says. “If that comes out, we could place the antibiotic and steroid in the eye and put the NSAID-eluting plug in the inferior puncta. Or, we could put the NSAID-eluting plug in the superior puncta and put a Dextenza plug in the inferior puncta. Either way, the procedure would be totally dropless, postop.”
Dr. Rowen adds that new lotepred-nol nanoparticle formulations are now available in drop form. “Those include Inveltys 1% ophthalmic suspension [Kala Pharmaceuticals] and Lotemax sub-micron ophthalmic gel 0.38% [Bausch + Lomb],” she says. “These have novel formulations and are designed to work for a longer time than previous modalities.”
Dr. Kershner points out that ophthalmologists—like people in general—tend to be resistant to trying new things. “It’s difficult to get doctors to change their habits, even when the proof that they should is staring them in the face,” he says. “Doctors like to make decisions based upon their clinical experience, but their clinical experience is often not based on real data. If surgeons have always done it a certain way and it seems to be working, then they don’t change. But ophthalmologists need evidence-based studies for doing what they do.
“Surgeons should be constantly re-evaluating their protocols to see if there’s a better way to do things,” he adds. “Review the literature. Go to the meetings. Look at what other surgeons are doing. Just because you’ve always done it that way doesn’t mean you should continue to do it that way.” REVIEW
Drs. Kershner and Mamalis report no financial ties to any product discussed. Dr. Rowen has consulted for Bausch + Lomb, Kala, Sun Pharma and Eyepoint. Dr. Matossian is a consultant for Eyepoint, Ocular Therapeutics, Ocular Sciences and Imprimis.



