Electroretinography is a test that measures the electrical activity generated by cells in the retina during exposure to a visual stimulus. Pattern electroretinography, or PERG, is a type of ERG that specifically measures the activity of retinal ganglion cells, which is of particular interest to doctors monitoring and treating glaucoma. PERG offers a few signifi-cant advantages over other tests that we routinely use to diagnose and monitor glaucoma patients. Most notably, PERG is able to detect functional abnormality very early in disease—in some cases possibly as much as eight years earlier than our other tests.1-7
Right now, many ophthalmologists don’t consider PERG to be standard-of-care, even though it’s been pos-sible to do this type of testing for decades. The problem has been that until recently the test was not very user-friendly; the equipment was cumbersome, and patients had to be hooked up to multiple sensors. As a result, instruments designed to do this type of testing were mostly put into corners at university practices and used by very specialized tech-nicians with PhDs. I believe most of us also found this technology off-putting when we were in training. Electrophysiology was a scary thing that most of us didn’t understand well, so we avoided it. For all of these reasons, few clinicians considered
using this technology in their private practice—at least until recently.
Now, all of that is changing. The instruments are becoming much more user-friendly, and the information uncovered with this technology is turning out to be very practical, including for those of us who are treating glaucoma. As already noted, PERG may be able to detect a problem at a very early stage—early enough to possibly treat it before ganglion cells die.
PERG and Glaucoma
The idea of using PERG testing for glaucoma suspects or patients is new to some ophthalmologists. Doctors tend to think of PERG as something you use when you’re trying to understand some obscure retinal dystrophy or brain problem. In addition, most of us still picture it as a cumbersome test. And of course, we’re inundated with technology, making it harder to appreciate the value of something that’s not being used by most ophthalmologists. Although I was skeptical when I first considered using it, I now believe in its ability to help my glaucoma patients.
Several companies offer instruments that can do PERG testing, including LKC Technologies, Konan Medical and Metrovision; we are currently using the Diopsys system. (I am a consultant for Diopsys.) We perform the test in a separate room. It only takes a few minutes for our technicians to set up and begin the test. We place two sensors on the patient’s face; one under the eye being tested on the lower lid, the other on the center of the forehead. The sensors are disposable, so there is a cost per procedure, but this is a reimbursable test, in the range of a little more $100 per test, so you’ll be able to recoup the cost of purchasing the equipment and providing the disposable sensors. Once the sensors are in place, the patient sits for a few minutes and watches the stimulus pattern playing on the monitor. We test one eye at a time.
It takes about 20 minutes to run the entire test. In addition to providing raw numbers as a score, the instrument compares the results to a normative database, making it much easier to decide if a result is abnormal than it was with previous versions of the technology.
PERG as a Tie-breaker
The primary way I use PERG today is as a tie-breaker when other test results are borderline, especially in glaucoma suspects. Deciding to treat isn’t a choice we should make lightly. On the one hand, we know the risk of progression in glaucoma suspects is reduced if we lower the intraocular pressure. On the other hand, glaucoma drops can be inconvenient and expensive, and once we start treatment we’re not going to stop, so the patient will be on treatment for a very long time. If someone definitely has glaucoma, putting him on drops is an easy decision, but many glaucoma suspects never develop glaucoma. That’s why if all the tests look normal and the normative database analysis agrees, we continue to watch the patient. On the other hand, if the results are borderline, PERG can help the clinician make a decision by revealing whether the retinal ganglion cells are functioning normally.
For example, one of my older patients presented with early cataract, suspicious optic nerves, a disc hemorrhage and borderline eye pressure and visual fields—a fairly typical glaucoma suspect. The right eye had a hint of a nasal step that corresponded to the location of the disc hemorrhage, and there was an indication of some abnormality on the visual field pattern deviation plot, but I didn’t find this evidence compelling. Furthermore, an OCT of his nerve fiber layer was within normal limits. In many cases like this one, in the absence of any other information, I’d postpone treatment and have the patient return in six months.
However, because the results were borderline, we ran the PERG test. The function in his right eye—the eye that had the disc hemorrhage—was worse than the left, and both eyes had borderline decreased magnitude, which is the thing I consider most relevant. That told me that his retinal function was wavering, and that made me decide to proceed with treatment instead of waiting. Because his disease was at a very early stage, most of his retinal ganglion cells were likely to still be alive, so treatment in this situation could actually produce some improvement in vision.
Other PERG Advantages
There are a number of other reasons for doctors treating glaucoma to consider adding the PERG test to their armamentariums:
• PERG measures the function of living cells that can still recover. Traditionally, when patients who are at risk come to us, we tell them we’re not going to treat them until they lose vision or until we measure a sign of optic nerve fiber layer loss on OCT; we don’t do anything until the patient gets worse. However, unlike the other tests we rely on, PERG measures the function of living cells. When the cells are in trouble, the conduction of electrical stimuli back to the brain becomes abnormal.
Finding that abnormality allows us to treat the patient much earlier—often, before the cells die. Some small-scale, preliminary studies have shown that the visual evoked potential of stressed cells may recover instead of dropping off if we then treat the patient.8-13 (Of course, that won’t always be the case, because the PERG test will be abnormal if the cells are already dead. In that case, treatment won’t bring them back.)
In contrast, if a white-on-white visual field reveals a defect, that segment of your vision is toast; the cells there are already dead and they’re not coming back, no matter what we do. At that point, the best we can do with glaucoma therapy is stop things from getting worse. It makes far more sense to diagnose and treat people before they have changes that are irreversible. PERG may give us a way to do that.
• PERG can be used to monitor the patient’s progress over time. This is a test that can be done serially. If it comes up normal but you have reason to be suspicious, you can check it once a year or so and look for change.
• PERG may indicate a problem when an angle is narrow but other tests appear normal. Sometimes the PERG test results will be abnormal in a patient who has narrow angles but has normal pressure when checked in the office. That suggests that the angle may be closing when it’s dark and the pupil dilates, spiking the pressure up. If PERG indicates that function is abnormal in a narrow-angle patient, you can do a laser iridotomy to keep the angle more open. I’ve done this in some patients, and I’ve seen the PERG results normalize at follow-up visits.
• PERG is sometimes helpful as a means to differentiate glaucoma from other problems with the macula, such as diabetic retinopathy. Glaucoma is most likely to be the problem affecting retinal ganglion cells; retinopathies tend to affect rods and cones. (That type of damage would be detected using a different test.) Certainly, when you’re faced with a patient who has a family history of glaucoma and a normal macula under examination, any abnormality picked up by PERG can be attributed to glaucoma until proven otherwise.
• The test is easy for the patient. The patient sits in front of the monitor at a set distance and watches the screen. This stands in contrast to visual fields where the patient has to respond to stimuli by pressing a button. Patients report that the PERG test is not unpleasant; you just have to sit there and pay attention.
• PERG is useful as a patient- education tool. Measuring the subtle changes in retinal ganglion cell function gives us a way to impress patients with the reality that using the therapies we prescribe makes a real difference, even though the drops are inconvenient and the patient can’t always tell that a potentially blinding process is taking place inside his eyes. In many patients, PERG will let you show the patient that when you lower the eye pressure, visual function improves. That can go a long way toward improving compliance with your therapy.
The Next Step Forward?
Early glaucoma patients and glaucoma suspects make up as much as 80 percent of the glaucoma patient population in most practices, and I’ve found PERG to be most useful with them. Once someone has more advanced disease, the horse is out of the barn; we know the patient is abnormal and we know what we’re going to do. On the other hand, at the early stages of the disease we need all the help diagnosing and monitoring that we can get, and PERG may give us a chance to catch decreased function early. (A variation on this technology such as visual evoked potential, or VEP, might be helpful when a patient has more advanced disease, but that’s another story.)
Although I think PERG is useful for the clinician, I believe we’re currently at a point with PERG that’s similar to where we were about 15 years ago with optical coherence tomography. Clinicians weren’t sure if OCT was necessary, and there were no user-friendly instruments like the ones we have today. The first OCT machines I saw in Boston in the 1990s took two MIT graduates to operate, and it took an hour to get one OCT scan. It was an interesting research tool, but definitely not practical for use in a clinic. However, over time OCT got faster and more user-friendly, and the manufacturers began developing normative databases. As the technology improved, people got more comfortable with it and saw the utility of it for detecting change.
Electroretinography has been around for 50 years—much longer than OCT—but in the past it wasn’t user-friendly enough that you would want to use it in a clinic, much less for glaucoma patients. If you used it at all, you’d reserve it for cases where you had no idea what the problem was. Today, that’s changing. Now, if I were suspicious that my own parents were developing glaucoma, I’d make sure they had this test.
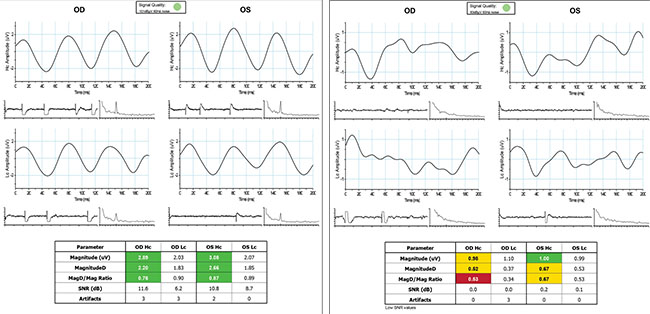 |
| Test results from a healthy patient (left) and a patient with glaucoma (right). The colored blocks indicate readings that fall within the normal range or are abnormal. |
Counterpoint: Dr. Medeiros
I believe that electrophysiology-based tests have enormous potential as methods for objective assessment of visual function in glaucoma, potentially helping us improve the way we diagnose and monitor the disease. However, I think this potential remains currently untapped due to the limitations of the commercially available devices.
A critical review of the evidence for the benefit of PERG-based tests in glaucoma management reveals that existing approaches still do not offer significant additional benefit compared to the standard of care based on standard automated perimetry and structural imaging with optical coherence tomography.
Reviewing the Evidence
A study from 2006, for example, investigated whether PERG could be used to predict which patients with ocular hypertension would convert to glaucoma during follow-up.3 The authors followed 95 eyes of 54 patients with ocular hypertension for an average of approximately eight years, performing PERG testing every six months. Of the 95 eyes, eight converted to glaucoma. Although the authors concluded that PERG helped predict conversion to glaucoma, a critical analysis of the results suggests a very poor predictive performance. At three years before conversion, areas under the receiver operating characteristic (ROC) curve for PERG parameters were all close to 0.5 or 0.6, too low to indicate any substantial prognostic value. A test that has an ROC curve area of 0.5 is like flipping a coin. Even at one year before conversion, PERG had a receiver operating characteristic curve area of only 0.78 for discriminating eyes that converted from those that did not, for a sensitivity of 80 percent and specificity of only 71 percent. It’s important to note that, because glaucoma is a slowly progressive disease, one year is really a short period of time in the course of the disease, and most patients with ocular hypertension do not develop glaucoma. Therefore, such performance seems unsatisfactory. It’s just not worth performing an additional test that will incur significant additional cost and burden to a subject if it will only give us perhaps a one-year advantage compared to perimetry in subjects with ocular hypertension.
Another study claimed that PERG-based testing was able to detect progressive RGC dysfunction as much as eight years earlier than optical coherence tomography in glaucoma suspects.1 However, this claim was based on comparing the technology to an obsolete version of OCT: time-domain OCT. Time-domain OCT requires large amounts of data interpolation due to its limited speed. More importantly, it lacks an accurate method to ensure image registration over time, causing large variability in measurements and limiting its potential for follow-up. This technology has been superseded by spectral-domain and swept-source OCT, which are much better tools for longitudinal monitoring of structural damage in glaucoma.
In the study, even though glaucoma suspects were followed over time with PERG and time-domain OCT, the authors do not report on the proportion that actually converted to glaucoma, using accepted endpoints such as visual field loss on standard perimetry. Therefore, it’s not possible to extract from the study the real value of PERG in predicting those who developed clinically significant outcomes. The figure suggesting that PERG would detect damage eight years earlier than time-domain OCT is based on analysis of the dynamic range and change seen in these tests during the study. However, as pointed out above, both the dynamic range and ability to detect change with time-domain OCT are quite limited.
Therefore, current evidence is still lacking on the real benefit that PERG-based testing would have in complementing the tools we currently employ in standard clinical care of glaucoma patients. It’s important to note, however, that the hypothesis that RGC dysfunction could be detected by electrophysiological methods before actual structural loss is picked up by OCT is a very sound and potentially impactful one. It just seems that currently available devices and technologies are still not making the best use of the potential for electrophysiology in glaucoma.
The Extra Test Factor
It’s important to note that adding another test in the follow-up of glaucoma patients or those suspected of having the disease incurs significant burden both to the patient and the health-care system. It becomes hard to justify such an additional burden unless the new test is shown to significantly improve patient outcomes, by which I mean a diagnosis or detection of progression that will truly have an impact on the bottom line—how the disease impacts quality of life. A test that is useful only in a minority of patients by giving just a small lead time in diagnosing damage is unlikely to have a clinically significant impact.
As I mentioned before, I do believe the potential for electrophysiology-based tests in glaucoma, as a means to provide us with objective assessment of functional status in the disease, is enormous. However, the greatest benefit of such tests will come if we can make them less cumbersome and more portable, allowing home-based testing. This would enable follow-up testing to be done at home and make a much larger number of test results available to help us detect change over time. REVIEW
Robert J. Noecker, MD, MBA, is in private practice at Ophthalmic Consultants of Connecticut in Fairfield, and is an assistant clinical professor at Yale University School of Medicine. He is a consultant to Diopsys.
Dr. Medeiros is a professor of ophthalmology and the Ben and Wanda Hildyard Chair for Diseases of the Eye at the University of California San Diego. He is also director of the Visual Performance Laboratory at UCSD.
1. Baritt MR, Ventura LM, Feuer WJ, et al. Progressive loss of retinal gangion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci 2013;54:2346–2352.
2. Bayer AU, Erb C. Short wavelength automated perimetry, frequency doubling technology perimetry, and pattern electro-retinography for prediction of progressive glaucomatous stan-dard visual field defects. Ophthalmology 2002;109:5:1009-17.
3. Bach M, Unsoeld AS, Philippin H, et al. Pattern ERG as an early glaucoma indicator in ocular hypertension: A long-term, prospective study. Invest Ophthalmol Vis Sci 2006;47:11:4881-7.
4. Ventura LM, Golubev I, Lee W, et al. Head-down posture induces PERG alterations in early glaucoma. J Glaucoma 2013;22:3:255-64.
5. Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci 2006;47:9:3904-11.
6. Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 2006;113:2:216-28.
7. Bode SF, Jehle T, Bach M. Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci 2011;52:7:4300-6.
8. Parisi V, Colacino G, Milazzo G, Scuderi AC, Manni G. Effects of nicergoline on the retinal and cortical electrophysiological responses in glaucoma patients: a preliminary open study. Pharmacol Res 1999;40:3:249-55.
9. Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology 2005;112:1:20-7.
10. Falsini B, Marangoni D, Salgarello T, et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: a short-term study by pattern electroretinogram. Graefes Arch Clin Exp Ophthalmol 2009;247:9:1223-33.
11. Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5’-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol 2005;110:1:91-102.
12. Ventura LM, Venzara FX 3rd, Porciatti V. Reversible dysfunction of retinal ganglion cells in non-secreting pituitary tumors. Doc Ophthalmol 2009;118:2:155-62.
13. Ventura LM, Feuer WJ, Porciatti V. Progressive loss of retinal ganglion cell function is hindered with IOP-lowering treatment in early glaucoma. Invest Ophthalmol Vis Sci 2012;53:2:659-63.



