Proliferative vitreoretinopathy remains an important cause of blindness in vitreoretinal practices. The opportunity to reattach the retina in even the most severe cases has been made possible by contributions such as wide-angle viewing and liquid perfluorocarbons. Despite these important advances in vitreoretinal surgical techniques, PVR remains the principal cause of blindness after retinal detachment. Surgical success rates for PVR vary from 65 to 90 percent depending on the severity of disease and the particular trial.1
However, visual outcomes are often poor due to macular involvement of the recurrent detachment. Hypotony, caused by ciliary body damage and increased outflow from large retinotomies, is also difficult to manage and can only temporarily be mitigated with silicone oil tamponade.1 Long-term tamponade and volume replacement is not feasible because of the toxicity of silicone oil.
 |
Prevention Over Treatment
Pharmacotherapy for PVR is an exciting area of research because the need for agents to reduce or eliminate proliferation continues to be critical in spite of advances in surgical technique. Treatments, which decrease or prevent recurrence in patients with established PVR, are important, but the best treatments will prevent the onset of PVR in patients with primary retinal detachment and high-risk characteristics associated with PVR development.
The classic example of a "doomed" eye is the post-ruptured globe with subretinal hemorrhage, in which the stimulus for PVR formation is almost certain despite successful reattachment of the retina. We are hopeful that someday even this patient could be treated to prevent PVR and recurrent retinal detachments.
The pathophysiology of PVR is complex and has several stages in its onset and progression.2,3 Inflammation is the first important factor in the cascade of events leading to neomembrane formation. Several factors increase the severity of inflammation including the number or magnitude of: retinal breaks, cryotherapy, liberated retinal pigment epithelial cells, surgical incisions or ruptures, and vitreous or subretinal hemorrhage. Inflammation causes the release of growth factors and cytokines, which lead to cellular proliferation.
Proliferating cells migrate, lay down collagen and can modulate scar tissue, creating retinal detachment.
No one growth factor stands out, but many, such as basic fibroblast growth factor (bFGF) and platelet derived growth factor (PDGF), have been implicated in the cellular proliferation of PVR.4,5 The movement of proliferating cells has been demonstrated in vitro, and heparin has been shown to decrease the ability of these cells to "stick" to the scaffold upon which they move.6
 |
Pharmacotherapy has focused on treating inflammation, cell migration, and cell proliferation. Corticosteroids decrease inflammation and stabilize the blood-ocular barrier. Dexamethasone has been used both in the vitrectomy infusate and as a depot injection at the end of PVR surgery. In addition, intravitreal triamcinolone acetonide (Kenalog) has shown some promise in reducing reproliferation after PVR surgery. Heparin decreases the "stickiness" of migrating cells and is typically used in the vitrectomy infusate for "high-risk" retinal detachment cases. We use a combination of 0.25 mL of dexamethasone phosphate (4 mg/mL) and 0.5 mL heparin sodium injection (1000 USP Units/mL) injected into a 500 mL BSS Plus (Alcon Inc.) bottle as the infusate in PVR vitrectomy as well as "high-risk" cases. A pilot study in 62 patients with severe PVR undergoing vitrectomy by a single expert PVR surgeon supports this method.7 All patients randomly received the combination of heparin (Pharmacia Inc.) and dexamethasone (Pharmacia Inc.) or control.
There was only a minimal increase in postoperative bleeding in patients receiving the combination therapy, but bleeding was not associated with an increase in redetachment or alteration of visual outcome. In fact, the combination therapy was associated with less reproliferation and less redetachment.
Next Target
Cellular proliferation is the next important therapeutic target in patients at risk for PVR. Chemotherapeutic agents such as methotrexate, daunomycin, and 5-FU, as well as radiation, have shown limited success in small clinical trials. The fear of cytotoxicity to the retina, optic nerve and ciliary body limit the practicality of widespread use of these agents, however. It is also difficult to titrate a safe and effective dose of drug as there is a narrow therapeutic index for these agents. Targeted growth factor treatment has not been attempted clinically and may be difficult because of the number of implicated growth factors and the high probability that multiple targets will be necessary to control proliferation.
 |
Immusol, Inc. (San Diego) has developed a chimeric ribozyme to proliferating cell nuclear antigen (PCNA), a critical cell-cycle division protein. We have demonstrated the efficacy of this molecule in the prevention of PVR in a rabbit dispase model of PVR.8-10
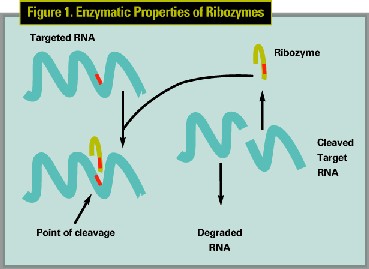
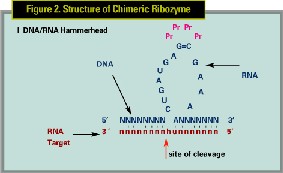
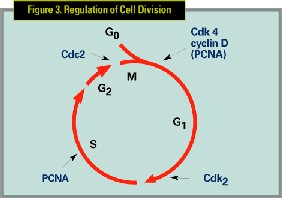
Ribozyme technology is promising because of the ability to target all implicated proliferating cells with a treatment, which is cytostatic rather than cytotoxic, thereby decreasing side effects. Ribozymes are small RNA molecules or oligonucleotide sequences that bind and cleave a specific sequence in the targeted mRNA molecule. (See Figures 1,2) Cell division is controlled by an array of proteins, including such cell division factors as cyclins and cyclin-dependent kinases. PCNA is one of several critical cell-cycle division proteins involved in the regulation of cell division. (See Figure 3)
Animal Studies
In the dispase-induced rabbit model for PVR, the ability of PCNA ribozyme to prevent or inhibit development of PVR and RD was tested. Experimental groups receiving intravitreal PCNA ribozyme, with or without a lipid vehicle, were compared to placebo-treated control groups. PVR progression in rabbit eyes was followed by indirect ophthalmologic examination and observations documented by fundoscopic photography, gross pathology and histopathology.
The chimeric ribozyme targeted a specific sequence in the rabbit PCNA that was found to be identical to that in the human. In vitro cleavage assays confirmed the ability of the ribozyme to cleave the mRNA of PCNA. In vitro studies with fluoresceinated ribozyme indicated that lipid vehicles facilitated delivery of the ribozyme into cells causative of PVR (RPE and fibroblasts), however, the PCNA ribozyme decreased the proliferation of fibroblasts, with or without a lipid vehicle. The ribozyme displayed good stability in vitreous fluid, whereas, it degraded quite rapidly in serum.
In animal experiments, rabbits in placebo-treated groups usually developed severe PVR characterized by focal traction or RD. Animals in the PCNA ribozyme-treated groups usually did not develop a RD, or if they did, it was small and localized, or focal areas of traction developed that did not progress to the degree that the placebo-treated animal eyes did over the follow-up period.
The in vivo use of a lipid delivery vehicle resulted in a precipitate; however, an effective "naked" ribozyme dose was identified that did not cause this side effect. The efficacy was similar with or without lipid carrier coupled, and because there was no precipitation with the "naked" ribozyme, it was chosen for further study. Toxicity studies of PCNA ribozyme have shown no significant toxic effects with the doses chosen for clinical study. Animal studies have shown inflammation with repeated injections of high doses of drug as has been shown with other oligonucleotide molecules.
Clinical Trial
A Phase 1/2 clinical trial, sponsored by Immusol, Inc., assessing the safety and efficacy of PCNA ribozyme (code name VIT100) to prevent recurrence of PVR is in progress. It is a randomized, double-masked, placebo-controlled multicenter trial (18 sites) which will enroll 150 patients with Grade C or worse PVR who are undergoing vitrectomy for retinal detachment repair. In the study, after the retina is completely attached, a single intraoperative, intravitreal injection of drug or placebo is given. The primary efficacy variable is the failure rate of retinal reattachment surgery due to PVR; all recurrent detachments are considered study failures. The study is anticipated to complete enrollment this winter, and six-month safety results are expected by the summer of 2004.
Modern surgical techniques have given hope to patients with severe retinal detachment complicated by PVR. Prevention and treatment of PVR can be minimized with modern instrumentation and advanced surgical technique. We use a combination of heparin and dexamethasone in the infusate for all PVR cases as well as "high-risk" primary detachments. We acknowledge that the best treatment for PVR would be at the earliest stages, perhaps even prevention of redetachment. Pharmacologic treatments alone provide the only possibility for this high goal. Molecular therapies such as VIT 100 have the potential to treat diseases such as PVR in a safe and effective fashion. If the safety of VIT100 is established, a large phase 3 trial will be necessary to prove its efficacy in this disease.
Dr. Mandava practices at Rocky Mountain Lions Eye Institute University of Colorado Health Sciences Center, 1675 N. Ursula St., Aurora, CO 80045. Contact him at (720) 848-2066, fax: (720) 848-5014 or e-mail: naresh. mandava@uchsc.edu.
1. Lewis H, Aaberg TM, Abrams GW, McDonald HR, Williams GA, Mieler WF. Subretinal membranes in proliferative vitreoretinopathy. Ophthalmology 1989;96:1403-1414.
2. Glaser BM, Lemor M. Pathobiology of Proliferative Vitreoretinopathy. St. Louis: Mosby, 1994:2249-2263.
3. Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci 1990;31:14-28.
4. Campochiaro PA, Glaser BM. Platelet-derived growth factor is hemotactic for human retinal pigment epithelial cells. Arch Ophthalmol 1985;103:576-579.
5. Wiedemann P. Growth Factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol 1992;36:373-384.
6. Glaser BM, Cardin A, Biscoe B. Proliferative vitreoretinopathy: The mechanism of development of vitreoretinal traction. Ophthalmology 1987;94:327-332.
7. Williams RG, Chang S, Comaratta MR, Simoni G. Does the presence of heparin and dexamethasone in the vitrectomy infusate reduce reproliferation in proliferative vitreoretinopathy? Graefe's Arch Clin Exp Ophthalmol 1996;234:496-503.
8. Mandava N, Blackburn P, Paul DB, Wilson MW, et al. Ribozyme to proliferating cell nuclear antigen to treat proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 2002;43:3338-3348.
9. Frenzel EM, Neely KA, Walsh AW, Cameron JD, Gregerson DS. A new model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1998;39:2157-2164.
10. Fastenberg DM, Diddie KR, Dorey K, Ryan SJ. The role of cellular proliferation in an experimental model of massive periretinal proliferation. Am J Ophthalmol 1982;93:565-572.