It is estimated that 15 million Americans have age-related macular degeneration. About 80 to 90 percent of affected people have the atrophic, or dry, form of the disease. The remainder have the exudative, or wet, form, which is characterized by the development of choroidal neovascularization (CNV). Although both forms can cause loss of vision, the exudative form is the cause of most of the cases of loss of vision due to this disease, and most research time and money have been devoted to studies of exudative AMD.
However, atrophic AMD can also cause loss of vision, and, more significantly, can progress to exudative AMD with its much greater risk for visual loss. Researchers are therefore also investigating regimens for slowing or preventing atrophic AMD.
Since the diagnosis and treatment of retinal and vitreous diseases began to emerge as a subspecialty of ophthalmology, laser photocoagulation has been the mainstay of treatment. In the past several years, however, there has been a rapidly increasing interest in the pharmacological treatment of AMD. These treatments have the potential to prevent vision loss due to AMD without the adverse effects that result from laser treatment.
Atrophic AMD
Dry macular degeneration is the most common form of the disease, accounting for up to 80 percent to 90 percent of cases of macular degeneration. In addition, the vast majority of patients with wet AMD at some point had dry AMD prior to the formation of CNV. Recently, the results of the Age Related Eye Disease Study (AREDS) demonstrated that taking high-dose supplementation with vitamin C, vitamin E, beta-carotene, and zinc can significantly lower the risk of visual loss in patients with moderate to advanced dry macular changes. The AREDS formulation showed a reduction in the risk of significant visual loss in these patients by about 25 percent.1 At this time, only these nutritional supplements have shown efficacy against non-exudative AMD. Clinical trials of laser therapy for certain eyes with drusen are well underway, but no conclusive results are yet available.
Exudative AMD
It is estimated that 1.2 million people in the United States currently have visual loss due to exudative AMD and that the disease causes 200,000 new cases of blindness a year. By the year 2030, there will be 88 million Americans over the age of 65. Since the incidence of AMD increases with advancing age, it is estimated that 6.3 million people will have visual loss due to exudative AMD and that the disease will cause 500,000 new cases of blindness a year. The curve steepens markedly beyond the age of 80, and, with more and more people living well into their 90s and 100s, exudative AMD will become epidemic, unless we curb the incidence of blindness with new approaches to prevent and treat this disease.
The exudative form occurs in only about 10 percent of people with AMD. However, the CNV that characterizes exudative AMD makes it responsible for 90 percent of the severe visual loss associated with the disease.
In the 1980s, the Macular Photocoagulation Studies (MPS) proved the benefit of laser photocoagulation treatment for extrafoveal and juxtafoveal CNV. Most patients, however, present with subfoveal CNV. More recently, the results of Treatment of Age-related Macular Degeneration with Photodynamic Therapy2,3 and the Verteporfin in Photodynamic Therapy4 investigations allowed retinal specialists to offer a treatment to patients with subfoveal CNV.
Again, however, this treatment is most likely to be beneficial in only a small percentage of patients, those with predominantly classic CNV, although a small but statistically significant benefit at two years has been shown to result from photodynamic therapy in some cases of CNV without classic fluorescein angiographic features.5 For the most part, however, the treatment options for most patients with CNV have been limited.
The AREDS also demonstrated that the high-dose dietary supplements used in the study reduced the risk of visual loss in patients with exudative AMD. However, most research today is directed at drug therapy. Many clinical trials, each with its own rationale, have been performed with the hope of finding safe and efficacious pharmacological treatment of exudative AMD. Some current drugs, such as the anti-VEGF aptamer Macugen of EyeTech Pharmaceuticals, the rhuFab antibody Lucentis from Genentech, and anecortave acetate from Alcon Labs, show promise. Clinical trials are also evaluating the treatment of subfoveal choroidal neovascularization with intravitreal corticosteroids, including triamcinolone acetonide (Kenalog), fluocinolone acetonide (Retisert), and dexamethasone (Posurdex). Other, previously studied drugs have already been proven ineffective. These include interferon, thalidomide, and a matrix metalloprotease inhibitor.
We have been privileged to participate in almost all of the clinical trials discussed in this review. Our practice has been fortunate to lead the United States' enrollment in all three phases of EyeTech's Macugen studies and in two anecortave acetate clinical trials. Our considerable experience in these studies has led us to be quite optimistic about the future of pharmacotherapy for the treatment of neovascular AMD.
Pegaptanib Sodium
Pegaptanib sodium aptamer (Macugen, EyeTech Pharmaceuticals) is an oligonucleotide that acts like an antibody with a high affinity for vascular endothelial growth factor (VEGF), preventing its uptake by VEGF receptors on endothelial cells.
The preliminary results from clinical trials were quite promising. Initially, a Phase I study of a single injection demonstrated the safety of the drug. Subsequently, a Phase II study was done in two parts, both with multiple injections. The study assessed safety as well as vision stabilization and/or improvement associated with the aptamer as a stand-alone treatment, as well as in combination with photodynamic therapy. The results showed that 87.5 percent of eyes that received Macugen alone had stabilized or improved vision three months after treatment and that 25 percent of eyes had three or more lines of improvement.6 In the group of patients who received both Macugen and photodynamic therapy, 60 percent had an improvement of three lines of vision at three months.
Anti-VEGF therapies block new blood vessel formation, but many people are unaware of the importance of the anti-permeability effect of these drugs. When we inject the drug into the vitreous, the retina gets thinner, and patients often experience an immediate improvement in vision. This probably has been significant in patients' acceptance of the treatment, since after improvement in vision they are happy to return for regular injections.
Macugen was granted fast-track designation by the Food and Drug Administration. Enrollment in clinical trials with patients with exudative AMD has now been completed, with about 1,200 patients enrolled through 117 investigational sites throughout the world.
rhuFab
Another inhibitor of VEGF is rhuFab (Lucentis, Genentech), a recombinant humanized anti-VEGF monoclonal antibody fragment. It is injected intravitreally in an office-based procedure similar to Macugen, except that Lucentis is injected every four weeks compared to Macugen's every six weeks. The Fab (antibody fragment) consists solely of the antigen-binding portion of the antibody and is smaller than the full antibody. It therefore should have improved retinal penetration.
In early testing on 53 patients with subfoveal CNVM, 50 (94 percent) had stable or improved vision at day 98 following intravitreal injection of Lucentis. The proportion of patients who had an improvement in vision equivalent to three or more lines of vision was 26 percent, a result almost identical to Macugen's.
Overall, the treated patients gained almost two lines of vision on average by day 98, compared with standard-of-care patients who lost one line of vision. There was a transient, mild to moderate inflammatory reaction following injections with Lucentis. (Heier JS, Sy JR, McCluskey ER, rhuFab V2 Study Group. rhuFab V2 in wet AMD—six month continued improvement following multiple intravitreal injections. Invest Ophthalmol Vis Sci;2002 ARVO E-Abstract #972)
These encouraging results have led to a Phase III trial of Lucentis for patients with predominantly classic subfoveal CNV. This study is evaluating the efficacy and safety of intravitreal injections of Lucentis administered monthly compared with that of verteporfin PDT administered every three months as needed. A second Phase III trial is also being conducted to compare Lucentis to placebo for patients with minimally classic/occult subfoveal CNV.
Anecortave Acetate
Anecortave acetate (Retaane, Alcon) is an angiostatic steroid. Because of modifications to its structure, it has no glucocorticoid activity and does not elevate intraocular pressure. Retaane inhibits extracellular matrix breakdown and endothelial cell division. It acts at several stages along the way in the development of new blood vessels, so it has the potential to be effective regardless of the etiology of the angiogenesis.
The drug is administered by posterior juxtascleral injection with a special applicator. Preclinical and clinical trials have provided promising results. In one study, patients were randomized to one of three different doses of retaane—3 mg, 15 mg, or 30 mg—or placebo.7 In the 15-mg treatment group, 79 percent lost less than three lines of vision. This was a better outcome than was seen in the other groups, including the 30-mg treatment group.
The 15-mg treatment group consistently fared better in other outcome measurements. Only 3 percent of patients in that group lost more than six lines of vision; the percentage in the other groups was much higher. In the subgroup of patients with predominantly classic lesions, 84 percent lost less than three lines of vision, and none lost six or more lines. This study has demonstrated that treatment with 15 mg of injected Retaane is far superior to placebo in maintaining positive visual outcome, preventing severe visual loss, and inhibiting lesion growth.
Corticosteroids
Several clinical trials have been evaluating steroid therapy for macular edema, diabetic and otherwise (See Figure 1). Corticoteroids are also known to act as anti-angiogenic compounds, and steroid therapy as an adjunct to PDT and even without PDT is now being considered for treating AMD.
 |
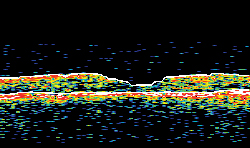 |
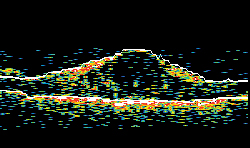
| Figure 1A. Ocular coherence tomography in a patient with diabetic macular edema, before intravitreal implant. Figure 1B. OCT in same patient, two months after intravitreal implant. The vision concomitantly improved from 20/125 to 20/63. | |
Small studies of triamcinolone acetonide (Kenalog) injected intravitreally for subfoveal CNVM have shown more stabilization or improvement in vision over the short-term compared with observation.8-11 The dose used in these studies was 4 mg and was generally well-tolerated. Up to 25 percent of patients may experience an increase of intraocular pressure, usually controlled topically, and there can be development of cataract, especially with repeat injections (personal data).
New York City ophthalmologist Richard Spaide has developed the idea of intravitreal Kenalog injections after PDT, and the treatment seems to have some potential benefit.12 Further clarification of the role of intravitreal Kenalog will be presented at next month's Academy of Ophthalmology meeting.
Another means of administering corticosteroids directly into the eye is with intravitreal implants. One such device is Bausch & Lomb's Retisert, a sustained-release implant that releases a low dose of fluocinolone acetonide into the vitreous over a three-year period (See Figure 2). Clinical trials are evaluating the implant in the treatment of diabetic macular edema, as well as subfoveal neovascularization in AMD. Twelve-month results in the diabetic macular edema trial showed that treated patients were more likely to have reduction in macular edema, less likely to have visual loss, and less likely to have worsening of diabetic retinopathy. Bausch & Lomb reports, however, that patients also were more likely to experience serious ocular adverse events, including increased ocular pressure, vitreous hemorrhage, and cataracts. No results have yet been reported in the AMD trials.
 |
| Figure 2. Retisert intravitreal implant. |
Another device, Oculex Pharmaceuticals Posurdex, is made of a biodegradable material (See Figure 3). The device is surgically implanted in the eye and slowly releases dexamethasone over approximately five weeks. The implant, which has an approximate weight of a sesame seed but is half its size, dissolves over time. Preliminary results of a Phase II clinical trial of treatment of diabetic macular edema showed that, 90 days after receiving the 700-microgram implant, patients experienced a statistically significant improvement in visual acuity, retinal thickness, and fluorescein leakage. Patients who received the 350-mcg Posurdex also showed statistically significant decreases in retinal thickness and fluorescein leakage, with a trend towards improvement in visual acuity. Oculex plans to initiate clinical trials in late 2003 to evaluate Posurdex in the treatment of CNV in AMD.
Gene Therapy
A Phase I clinical trial is underway to evaluate AdPEDF, a gene therapy product developed by GenVec, Inc. Pigment epithelium-derived factor (PEDF) is an antiangiogenic protein that is normally produced in the eye and plays a part in regulating angiogenesis. AdPDEF is a modified adenovirus that is designed to carry the PEDF gene into eye cells and stimulate them to produce PDEF, which would inhibit neovascularization.13,14
Other Treatments
Several pharmacological agents were considered to be possible treatments for AMD on the basis of preclinical and early clinical research. Subsequent clinical trials, however, showed that they did not work. These were given systemically, which may have been one of the reasons for failure, due to inability to reach adequate concentrations in the eye and to systemic side effects.
• Interferon. The story of the interferon-alpha 2A study is one worth remembering when analyzing new treatments for AMD. There was great initial enthusiasm, which resulted in extensive, favorable media coverage for this treatment even before any randomized trials had been done. Subsequently, a large international multicenter study was undertaken in a randomized controlled fashion. This failed to show any treatment benefit; in fact, the placebo group statistically did better than the treated group.15
• Thalidomide. Clinical and laboratory studies have demonstrated that thalidomide inhibits the growth of new blood vessels. A small study was performed to assess the effects of thalidomide on CNV and to determine whether a larger study should be performed. Nearly two thirds of the study subjects who were taking thalidomide stopped taking it because they were unable to tolerate the side effects, which included drowsiness, constipation, and peripheral neuropathy.
The researchers decided that, because the subjects in this small study were unable to tolerate the drug's side effects, a larger study should not be performed. (Maguire MG, Fine SL, et al, the AMDATS Research Group. Results of the Age-related Macular Degeneration and Thalidomide Study (AMDATS). Invest Ophthalmol Vis Sci; 42(4)ARVO Abstracts #1255.)
 |
| Figure 3. Posurdex intravitreal implant. |
• Metalloprotease (MMP) inhibitors. A study sponsored by Agouron Pharmaceuticals (subsequently Warner Lambert and then Pfizer) evaluated another type of drug, a metalloprotease inhibitor that inhibits blood vessel growth. The purpose of the study was to determine the effects of the drug on visual acuity and on the morphology of the CNV in people with AMD. The results showed that the drug was not effective in inhibiting the growth of CNV.
Perhaps the most exciting aspect of these emerging treatments for AMD is that different mechanisms are being investigated to treat this devastating disease.
One could easily imagine a multimodality approach, where a combination of treatments may be used. Already, there are studies of anti-VEGF intravitreal injections in conjunction with PDT using verteporfin. Also, feeder vessels appear to be more easily identified following a session of PDT, and so perhaps there will be a role for a combination of different laser treatments in addition to pharmacological treatments for exudative AMD.
Of course, the best treatment is prevention, and, since antioxidant vitamins and zinc, as shown in the AREDS, can reduce the risk of developing CNV, the impact on prevention of visual loss could be considerable. The Complications of Age-related Macular Degeneration Prevention Trial (CAPT) and other studies of laser treatment in selected eyes with drusen are nearing completion. If these prove effective, significant further reduction in the incidence of CNV and subsequent blindness would be forthcoming.
We are quite hopeful that the combination of preventive therapies, traditional and newer laser approaches, and new pharmacological treatments will result in a dramatic reduction in the incidence of blindness from AMD, despite the continued aging of the world's population. Certainly much work has yet to be done, but the future for treating AMD has never been more optimistic.
Drs. Singerman and Miller practice at Retina Associates of Cleveland. Dr. Singerman is a clinical professor of ophthalmology at Case Western Reserve University School of Medicine. Contact him at 3401 Enterprise Parkway, Suite 300; Cleveland, Ohio 44122; phone 216-831-5700; fax 216-831-1959; e-mail Lsingerman@retina-assoc.com. Joan Hornik of Retina Associates of Cleveland provided indispensable editorial assistance at every stage in the development of this review.
1. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol 2001; 119:1417-1436.
2. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol 1999; 117:1329-45.
3. Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials–TAP report. 2. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol 2001; 119:198-207.
4. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Verteporfin In Photodynamic Therapy Study Group. Am J Ophthalmol 2001;131:541-560.
5. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Verteporfin In Photodynamic Therapy Study Group. Am J Ophthalmol 2001;131:541-560.
6. Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003;110:979-986.
7. D'Amico DJ, Goldberg MF, Hudson H, Jerdan JA, Krueger S, Luna S, Robertson SM, Russell S, Singerman L, Slakter JS, Sullivan EK, Yannuzzi L, Zilliox P; Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for the treatment of subfoveal lesions in patients with exudative age-related macular degeneration (AMD): interim (month 6) analysis of clinical safety and efficacy. Retina 2003;23:14-23.
8. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 2000;20:244-250.
9. Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: 18-month follow-up. Aust N Z J Ophthalmol 1998;26:277-281.
10. Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol 1995;23:293-298.
11. Gillies MC, Simpson JM, Luo W, Penfold P, Hunyor AB, Chua W, Mitchell P, Billson F. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol 2003;121:667-673.
12. Spaide RF, Sorenson J. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Macula Society Abstracts 2003, pp. 94-95.
13. Rasmussen H, Chu KW, Campochiaro P, Gehlbach PL, Haller JA, Handa JT, Nguyen QD, Sung JU. Clinical protocol. An open-label, phase I, single administration, dose-escalation study of ADGVPEDF.11D (ADPEDF) in neovascular age-related macular degeneration (AMD). Hum Gene Ther 2001;12:2029-2032.
14. Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Duh EJ, Yang HS, Cingolani C, Lai H, Wei L, Campochiaro PA. Periocular injection of an adenoviral vector encoding pigment epithelium-derived factor inhibits choroidal neovascularization. Gene Ther 2003;10:637-646.
15. Interferon alfa-2a is ineffective for patients with choroidal neovascularization secondary to age-related macular degeneration. Results of a prospective randomized placebo-controlled clinical trial. Pharmacological Therapy for Macular Degeneration Study Group. Arch Ophthalmol 1997;115:865-872.



