Photodynamic Therapy
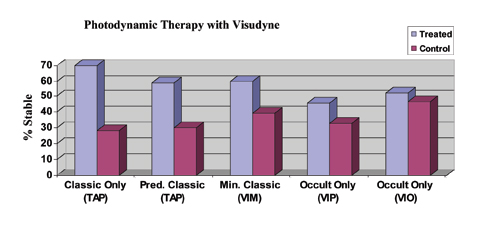 |
| Figure 1. Efficacy of PDT with Visudyne (verteporfin) for the treatment of subfoveal CNV. Stable is defined as the percentage of patients losing < 15 letters (three lines) of vision. All data are from the two-year results. Differences are statistically significant (p<0.05) for all studies except VIO, which reported no significant difference. Data from the VIM trial are Phase II only, while all other data is Phase III; Phase II data may not accurately reflect the more realistic expectations provided by Phase III study data. |
Photodynamic therapy with Visudyne (Novartis Pharmaceuticals) was approved for use by the Food and Drug Administration for predominantly classic CNV in April 2000 based on the TAP (Treatment of AMD with Photodynamic therapy) trial.1,2 The Verteporfin in Minimally Classic trial (VIM)3 and the Verteporfin in Photodynamic Therapy trial (VIP)4 support the use of PDT for treatment of minimally classic and occult lesions less than four MPS disc areas in size that have shown evidence of recent progression, respectively. Treatment of these select patients is not FDA-approved, but is currently covered by the Centers for Medicare & Medicaid Services. The FDA has approved PDT for the treatment of CNV in pathologic myopia and histoplasmosis. In each study, treatment was applied up to every three months if angiography showed persistent leakage. Early retreatment (six weeks) did not improve outcomes in the Verteporfin in Early Retreatment (VER) trial.5 Efficacy of PDT with Visudyne against the lesion subtypes at two years is summarized in Figure 1. Of the various CNV subtypes, Visudyne PDT as monotherapy appears to work best for predominantly classic disease. The evidence for efficacy of PDT in treating the occult-only subtype has weakened with the recently completed Verteprofin in Occult (VIO) trial, in which the study's primary visual outcome was not reached at either one or two years (not shown).
Visudyne is administered intravenously, and its mechanism of action involves preferential uptake of this photosensitizing dye that, when activated by specific laser light energy, selectively closes the CNV vessels by thrombosis. Side effects include transient visual disturbance (18 percent), injection site reactions (13 percent), and back pain (2 percent).1 Acute severe vision decrease (greater than four lines) occurred in 0.7 percent in the TAP study1 and 4.4 percent in the VIP study,3 and was more common in the larger occult lesions with good vision, which are generally not recommended for treatment.
Triamcinolone Acetate
Triamcinolone acetate has been used off-label for the treatment of CNV with the goal of utilizing its anti-inflammatory and antiangiogenic properties. Investigators initially reported improvement in vision and reduction in leakage at three months following treatment.6-8 A randomized, controlled study did not find benefit at one year for those patients receiving a single injection, however.9 Multiple injections of TA (three-month intervals) appear to benefit some patients,7 but no controlled data exists and, given the increased risks of secondary glaucoma and cataract progression as well as the new treatment options now available, TA alone is rarely used.
| Macugen |
 |
| Figure 2. Two-year data on the efficacy of Macugen for the treatment of subfoveal CNV in AMD. Stable is defined as the percentage of patients losing < 15 letters (three lines) of vision. Results are from the VEGF Inhibition Study In Ocular Neovascularization (VISION) trials. Predominantly classic: > 50 percent of the lesion is classic. Minimally classic: < 50 percent of lesion is classic and occult: no classic component. All data are statistically significant (p<0.05). |
PDT Plus Triamcinolone Acetate
The addition of triamcinolone acetate to PDT with Visudyne (PDT/TA) is not FDA-approved. PDT alone can close CNV, but also tends to cause a transient inflammatory response and increase in permeability along with a burst of VEGF activity shortly after treatment that may limit its efficacy. The addition of TA may reduce inflammation and inhibit VEGF activity. Corticosteroids also inhibit the formation of MMP-2 and MMP-9, the matrix metalloproteinases active in neovascularization.
A recent uncontrolled study of 26 patients reported a retreatment rate of 1.2 for the first year compared to the rate of 3.4 for the original TAP study.10 Patients without prior PDT therapy had a +2.5-line improvement of visual acuity, and those with a prior history of PDT experienced a +0.44-line improvement. Secondary elevated intraocular pressure occurred in 38.5 percent and was controlled with topical medication in all cases. Larger clinical studies are under way evaluating the combination of PDT/TA.
Macugen
Macugen was approved by the FDA in December of 2004 for treatment of all subtypes of CNV—predominantly classic, minimally classic and occult with no classic. The approval was based on the results of the VEGF Inhibition Study In Ocular Neovascularization (VISION) trials.11
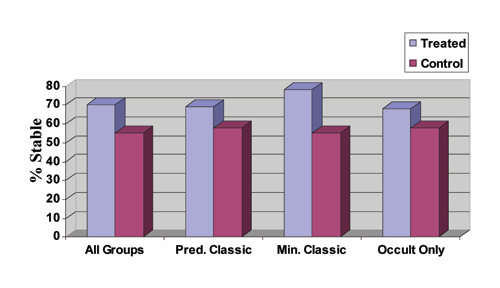
The two-year response data for all patients and all subtypes are shown in Figure 2. Note that no one subtype drove the results. In all cases, treatment was superior to usual care, which included the use of PDT with Visudyne for predominantly classic lesions at the investigators' discretion.
Macugen is a novel drug—a highly stable oligonucelotide fragment (aptamer) that selectively binds VEGF subtype 165, the form most responsible for ocular disease. Macugen is administered by intravitreous injection every six weeks for up to two years. At one year, Macugen reduced risk of progression to legal blindness by 50 percent; 70 percent of patients lost less than three lines of vision (versus 54 percent for the control); and the therapy was effective against all subtypes. In a separate study extending treatment for a second year, Macugen continued to provide a statistically significant benefit.
| "Stabilization" of Vision (<3 line loss) by Treatment |
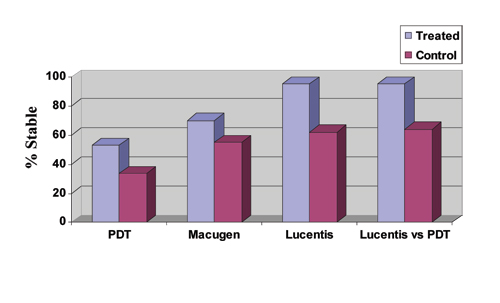 |
| Figure 3. PDT with Visudyne data is weighted average of two-year results from the TAP, VIM and VIP trials for treatment of predominantly classic, minimally classic and occult CNV in AMD, respectively, giving an average treatment effect for all subtypes. Macugen data is for all treatment subtypes at two years. Lucentis data is for both the 0.3 and 0.5 mg groups for treatment of minimally classic and occult subtypes at one year as reported for the Marina study, American Society of Retina Specialists Meeting, July 2005. Lucentis versus PDT data is for the treatment of predominantly classic subtypes at one year as reported for the ANCHOR study. All differences are statistically significant. Note again that the VIM study data are Phase II results and may not accurately reflect the efficacy of a Phase III trial. |
Lucentis (Ranibizumab)
Lucentis is still under clinical investigation. Lucentis is the antigen-binding portion (Fab fragment) of the full antibody bevacizumab (Avastin) with the addition of genetic engineering to increase the affinity for VEGF.12 Lucentis binds to all identified VEGF isoforms with the goal of maximal inhibition of angiogenesis and vascular permeability. Concerns about blocking the "normal" VEGF isoforms required to maintain the retinal or choroidal vasculature have been raised, but such side effects have not been identified to date.
Like Macugen, Lucentis is injected into the eye, only more frequently—every four weeks instead of every six weeks. The one-year results from the MARINA (Minimally classic/occult trial of the Anti-VEGF antibody Ranibizumab In the treatment of Neovascular AMD) trial comparing Lucentis to observation for minimally classic and pure occult CNV were promising, with "stabilization" of vision (<15 letter loss) in 95 percent of treated patients versus 62 percent of controls (0.3- and 0.5-mg doses). This "stabilization" of vision was much higher than other therapies, and this comparative data is summarized in Figure 3, although subgroup analyses will be needed to assess the applicability of these data to all AMD patients. A greater than three-line improvement in visual acuity was reported in 25 percent for the 0.3-mg group and in 34 percent for the 0.5-mg group compared to 5 percent in each control group. Ocular side effects included uveitis in 0.6 percent and endophthalmitis in 0.6 percent of eyes. The systemic safety profile to date appeared to be acceptable.
A more recent study, ANCHOR (ANti-VEGF Antibody for the Treatment of Predominantly Classic CHORoidal Neovascularization in AMD) compared Lucentis to photodynamic therapy with Visudyne for the treatment of predominantly classic CNV. Approximately 94 percent of patients treated with 0.3 mg of Lucentis, and 96 percent of those treated with 0.5 mg of Lucentis maintained or improved vision compared to 64 percent of those treated with Visudyne PDT (p<0.0001) during the first year of the two-year study. The Lucentis treatment groups further demonstrated a statistically significant difference from the control arm in an important secondary endpoint: mean change in VA from baseline to month 12. On average, the vision in patients treated with Lucentis improved, while patients treated with PDT declined.
Avastin: Administration
Avastin has now been tried off-label in an uncontrolled fashion for treatment of CNV. As noted above, Avastin is the full size anti-VEGF antibody from which Lucentis is derived. Intravenous administration of the drug has been tested in an uncontrolled, open-labeled pilot study in nine patients with subfoveal CNV from AMD.13 The authors administered the drug as approved for metastatic colon cancer: 5 mg/kg at two-week intervals, two doses per patient, and reported significant improvement in vision, macular edema and no significant side effects.
More recently, the same group of investigators reported a case of improved vision and reduced macular edema following intravitreous injection of Avastin for subfoveal CNV in a patient with neovascular AMD.14 This patient had failed initial treatment with several other modalities of therapy.15 Off-label use of Avastin has become increasingly popular, but guidelines for its use have yet to be established. Some specialists use the drug once and reassess at four to six weeks while others commit the patient to three consecutive injections at four to six week intervals and then reassess response to treatment with reinjections recommended at four to six week intervals if lesions still show persistent edema on OCT. Randomized controlled studies have been recommended to assess the safety and efficacy of intravitreous injections of Avastin for the treatment of macular degeneration, and a head-to-head study of Lucentis to Avastin is under consideration.
Other Agents under Evaluation
Another drug in the pipeline is squalamine (Evizon, Genaera), an antiangiogenic aminosterol originally derived from the dogfish shark liver and delivered via intravenous injection every four weeks. Preliminary phase I open label trial results vision stabilization or improvement out through three months of follow-up. A larger controlled Phase II trial is under way.
Anecortave acetate (Retaane) is another novel agent. Retaane is derived from a steroid and has been modified to reduce or eliminate glucocorticoid activities. The drug works intracellularly to presumably block matrix metalloproteinase (MMP) production, thereby inhibiting the vascular endothelial cell from proliferating and migrating. It is administered by periocular injection with a specialized cannula. Preliminary Phase II/III results looked promising for the 15-mg dose, but it failed to reach the noninferiority endpoint in a larger, Phase III head-to-head comparison to Visudyne PDT for treating predominantly classic CNV. Further studies are under way. The drug is also under evaluation as a prophylactic agent for high-risk patients with dry AMD (CNV in fellow eye).
Several other agents are in that pipeline, including intravitreous injection of the regulatory growth factor pigment epithelium-derived factor (PEDF) contained with an adenovirus vector. The adenovirus contains a gene cassette for PEDF. About 20 patients with advanced exudative AMD have participated in a dose and safety trial. The treatment appears to be safe and, although it's difficult to interpret visual acuity from patients with these advanced lesions, the fluorescein angiography data were promising. The trial is currently expanding with higher doses.
Acuity Pharmaceuticals is investigating the use of interfering ribonucleic acid (RNA) fragments that bind to a larger molecule called RNA-induced silencing complex (RISC). A selective fragment can degrade messenger RNA for VEGF, and thereby block the production of VEGF. Fragments also have the ability to modulate gene expression. One molecule of soluble ribonucleic acid (sRNA) potentially can inhibit the production of hundreds of VEGF molecules. Another preemptive strike is the VEGF trap by Regeneron. A clinical trial is under way.
The transpupillary thermotherapy (TTT) study for pure occult and minimally classic CNV did not meet the study's primary outcome of preventing moderate vision loss (three or more lines) However, in patients who were treated with TTT, there was a significant difference in those who improved three lines or more, compared to sham. In addition, a subgroup analysis of patients with baseline visual acuity of 20/100 or worse revealed an increasingly beneficial effect over the two-year study. No patients in the sham group showed improvement compared to 22 percent in the TTT-treated group.
Combination Therapy
The fight against macular degeneration will likely require a multi-pronged approach. Combination therapy including Macugen plus Visudyne, Macugen plus TA, Lucentis plus Visudyne and PDT plus TA, have been tried and some are under formal evaluation to assess efficacy and risks.
Both Macugen and Visudyne PDT are effective treatments for patients with wet AMD. In the VISION study, Macugen was superior to usual care, which included PDT for predominantly classic lesions at the investigators' discretion. A head-to-head comparison was not conducted, however, and data analysis suggests that for predominantly classic and small (greater than four disc areas) minimally classic lesions, the efficacy may be comparable. For larger occult and minimally classic lesions (less than four disc areas), only Macugen been shown to be effective.
The combination of Visudyne PDT and Macugen is currently under clinical study. The addition of triamcinolone acetate to PDT with Visudyne has not been evaluated in a controlled study yet. It appears to add the benefit of improved efficacy and reduced need for retreatment, but it also increases the risk of secondary glaucoma, cataract and endophthalmitis. The anti-VEGF antibody fragment, Lucentis, appears to be more efficacious overall than both Macugen and PDT with Visudyne for both pure occult and minimally classic lesions, but is not yet approved or available. Its efficacy against the more aggressive classic lesions has yet to be reported. The off-label use of Avastin has become increasingly popular for the treatment of all forms of wet AMD, but controlled safety and efficacy data are absent. Clinical trials for other agents include synthetic and natural steroids, other growth factors and nucleic acid derivatives that may interfere with synthetic pathways for VEGF. Enrollment in these studies is crucial to assess the potential benefit of these new therapies. A combination of therapeutic modalities may ultimately be required to achieve the greatest benefits for our patients.
Drs. Gallemore and Boyer are in private practice with the Retina-Vitreous Associates Medical Group in Beverly Hills, Los Angeles, North Hollywood and Torrance. They are clinical faculty at the Jules Stein Eye Institute, UCLA School of Medicine (RPG) and the Doheny Eye Institute, USC School of Medicine (DSB). They can be reached at (310) 854-6201 or via e-mail at Retina 2000@Yahoo.com (RPG) and vitdoc @aol.com (DSB). They are investigators in clinical studies for both EyeTech, Novartis and Genentech.
1. Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol 1999;117:1329-1345.
2. Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials—TAP Report 2. Arch Ophthalmol 2001;119:198-207.
3. Azab M, Boyer DS, Bressler NM et al. Visudyne in Minimally Classic Choroidal Neovascularization Study Group. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthalmol 2005;123:448-57.
4. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001;131:541-60.
5. Eter N, Vogel A, Inhetvin-Hutter C, Spitznas M. Interval reduction of photodynamic therapy (PDT) in age-related macular degeneration (AMD) is not advantageous. A pilot project. Ophthalmologe 2003;100:314-7.
6. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 2000;20:244-250.
7. Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF. Intravitreal reinjection of triamcinolone for exudative age-related macular degeneration. Arch Ophthalmol 2004;122:218-222.
8. Jonas JB, Degenring RF, Kreissig I, Friedemann T, Akkoyun I. Exudative age-related macular degeneration treated by intravitreal triamcinolone acetonide: A prospective comparative nonrandomized study. Eye 2004;25:1-8.
9. Gillies MC, Simpson JM, Luo W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: One-year results. Arch Ophthalmol 2003;121:667-673.
10. Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2005;112:301-4.
11. Gragoudas ES, Adamis AP, Cunningham ET Jr., Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351:2805-2816.
12. Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. IOVS 2004;45;E-Abstract 2276.
13. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: Twelve-week results of an uncontrolled open-label clinical study. Ophthalmology 2005;112:1035-47.
14. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical Coherence Tomography Findings After an Intravitreal Injection of Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration Ophthalmic Surg Lasers Imaging 2005;36:331-335.
15. Klesert TR (editor) So you want to try intravitreal Avastin. Retina Times 2005;14:18-21.



